Deep brain stimulation (DBS) is a modern therapy method from the field of functional neurosurgery, i.e. the branch of neurosurgery that modulates pathological brain functions in order to alleviate symptoms of neurological diseases. Research over the past 30 years has shown that DBS can be used to successfully treat many diseases. At Inselspital, a DBS procedure is routinely performed under general anesthesia and not as an awake operation. However, this requires a high level of expertise and a great deal of experience on the part of the treating physicians.
Before the actual surgery, a very good patient selection is the prerequisite for the success of the treatment. Not all patients are suitable candidates for DBS surgery.
The indication must be discussed and jointly made by neurologists, neurosurgeons, neuropsychologists and psychiatrists on an interdisciplinary basis. All potential candidates are discussed at Inselspital within the context of an interdisciplinary board. Here, the indication, advantages and disadvantages of certain treatment alternatives to DBS (focused ultrasound), the exact target point with the expected effects and side effects are discussed in the context of the patient's respective clinical picture and his individual needs. Criteria such as operability and other concomitant factors (e.g., neuropsychological findings, concomitant psychiatric disorders) are also considered.
Examinations before surgery
All patients are given a high quality MRI of the head prior to surgery. The MRI images are used to identify the precise anatomical target for DBS.
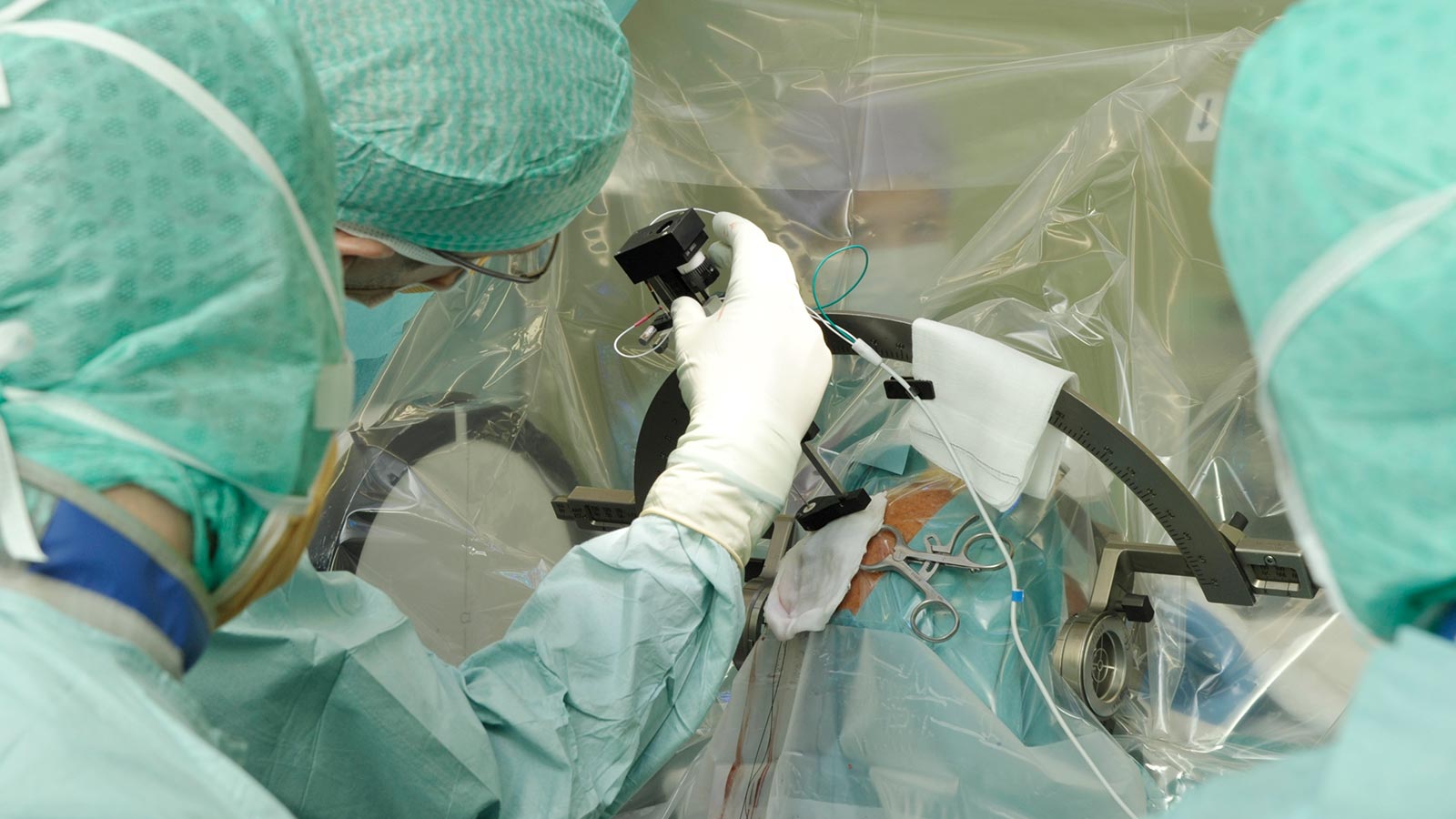
Stereotactic surgery
In most cases, surgery is performed using a stereotaxic frame. The attachment of the stereotactic frame to the patient's skull is usually performed under local anesthesia. The frame contains a coordinate system that is important for the precise insertion of the electrodes.
Once the stereotaxic frame is in place, imaging of the head is performed, usually a computed tomography (CT) scan. This "stereotaxic CT" is overlaid on the patient's MRI with a precise fit using software and is used to assign each point in the brain (selected on the MRI) to certain specific coordinates on the frame. These coordinates can later be set on the frame and allow the electrodes to be inserted to the desired target point with millimeter accuracy.
After the CT has been performed, the patient, accompanied by the surgeon and supervisor, goes to the operating room, where the procedure is performed under strictly sterile conditions.
At Inselspital: deep brain stimulation routinely performed under general anesthesia
The advantages are
- very precise implantation of the electrode
- no stressful awake operation
- shorter operation time
- gentler procedure for our patients
- no stopping of Parkinson's medication before surgery
The change from awake surgery to surgery under general anesthesia is based on the experience of major international centers and scientifically published results *. We have been recommending and practicing the implantation of electrodes and pacemakers under general anesthesia since 2021. In our experience, the results of surgery under anesthesia are just as good or even better than with awake surgery. However, this requires a high level of expertise and a great deal of experience on the part of the treating physicians. We have also found that all our Parkinson's patients prefer surgery under general anesthesia.
This innovative procedure has been made possible by
- the improvement in modern imaging, which allows a precise and accurate implantation of the electrode even under general anesthesia
- the technological advancement of the implanted systems (segmented electrodes).
As with awake surgery, electrophysiological signals from the brain are derived during the operation under general anesthesia and intraoperative test stimulation is also carried out to test for side effects. In this way, the placement of the electrode can be checked with millimeter precision. If brain signals are not derived and/or side effects occur rapidly, the electrode is repositioned and tested again.
If medically indicated, the procedure can also be performed as an awake surgery in individual cases. Our treatment team will discuss this with you in detail in advance and also explain the procedures for awake surgery, should this be necessary. In patients with severe dystonia, however, the procedure must always be performed under general anesthesia.
Which conditions can be treated with DBS?
The most frequent indications for DBS concern the area of movement disorders *, *, *. However, DBS is also used as a treatment alternative for severe forms of obsessive-compulsive disorders that do not respond to standard therapy. It is also a treatment option for chronic pain syndromes. In addition, DBS is currently being researched and clinically tested as an option for the treatment of other severe disorders.
Movement disorders
Obsessive-compulsive disorders
Chronic pain syndromes
Current research
- Alzheimer's dementia
- anorexia
- Huntington's disease
- multiple system atrophy (MSA)
- and many others
DBS continues to be the subject of intensive fundamental and clinical research. The number of diseases for which DBS is emerging as a potentially effective therapeutic measure is growing steadily. Continuous technical advancements in neuro-stimulators and stimulating electrodes, as well as improvements in medical imaging techniques, are also contributing to the immense development potential of this treatment modality.
Segmented DBS electrodes for directional stimulation
Until now, DBS electrodes available on the market consisted of 4 ring contacts arranged along the vertical axis of the electrode, generating a rather spherical electric field around the electrode (360°). Pioneering work by our group led to the development of a segmented electrode that allows directional stimulation.
Instead of generating a spherical electric field that spreads in all directions, segmented electrodes allow the electric field to be more targeted in space. This helps us to ensure that structures with a positive clinical effect (e.g., tremor suppression in Parkinson's disease or essential tremor) can be stimulated in a more targeted manner. At the same time, structures whose stimulation is associated with undesirable side effects (e.g., paresthesias or speech disorders) can be better avoided. Initial studies have shown that this theoretical concept also works in practice *, *.
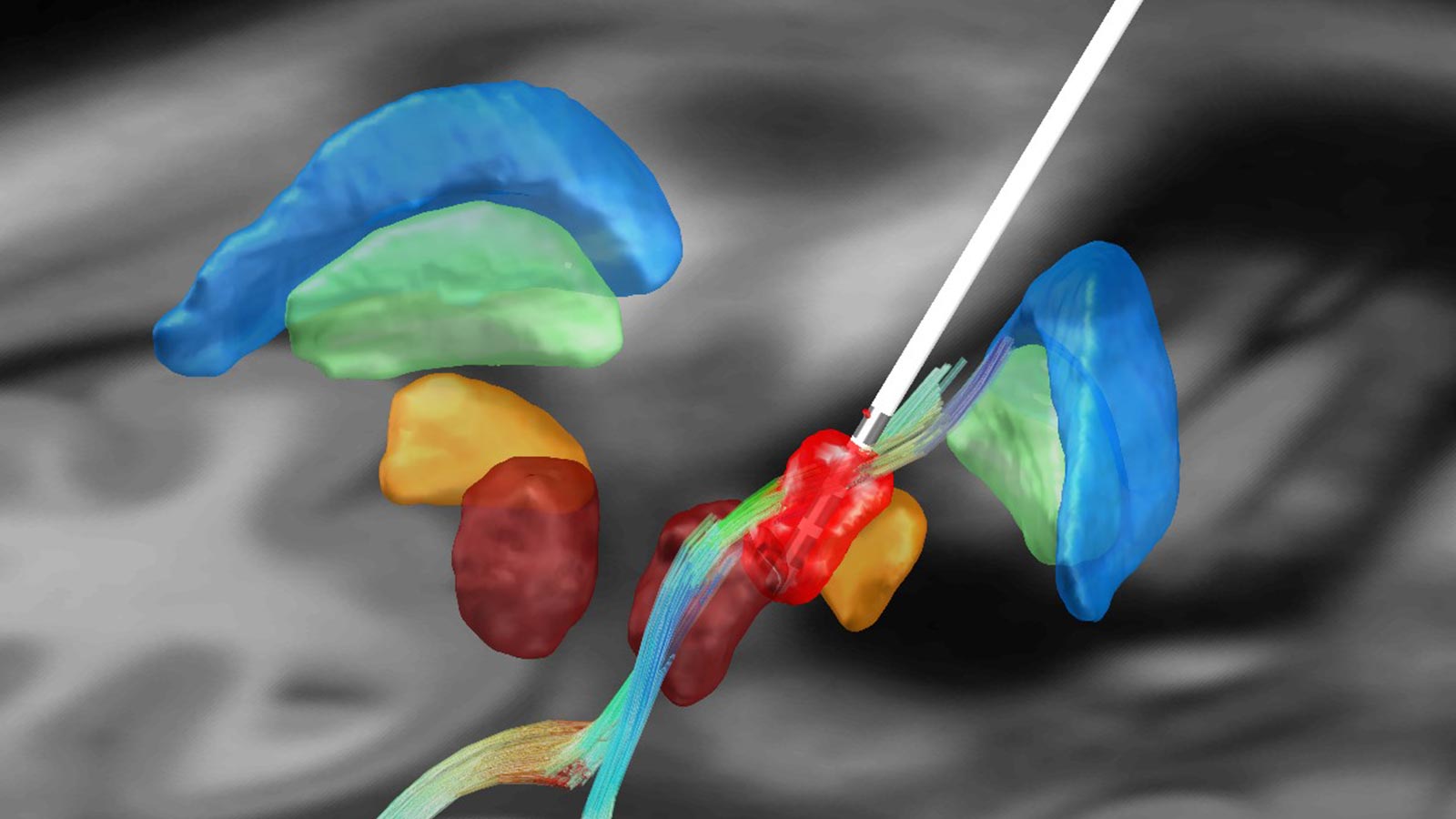
Patient-specific planning using tractography for tremors
During DBS, the classic target for the treatment of tremor is the motor thalamus (ventralis intermedius nucleus or VIM). However, on conventional MRI images, the thalamic nuclei are not separable and identifiable. Therefore, to plan the VIM, one combines MRI imaging of the patient with classical stereotactic coordinates, which have proven to be historically effective averages for targeting the VIM.
Modern imaging techniques allow approximate visualization of anatomical fiber bundles. These imaging techniques, based on mathematical models, are commonly known as diffusion tensor imaging (DTI). Research from other research groups as well as our own group suggests that stimulation of the so-called cerebellothalamic tract (CTT) or dentatorubrothalamic tract (DRTT) is responsible for the tremor suppressing effect *, * *, *.
Enabled by modern imaging techniques such as DTI, the fiber tracts can be modeled using the process of tractography. An individual, patient-specific planning of the optimal target point for the treatment of tremor thus becomes possible.
Simplification of postoperative programming
The development of a new generation of segmented electrodes that allow the electric field to be directed specifically in a desired direction is promising in terms of expanding therapeutic options. Initial pilot studies demonstrate this. However, the additional possibilities of programming these electrodes also place great demands on the treating physician and the patient. The empirical approach in finding the best stimulation parameters is extremely time consuming and can take several hours per test.
One of our research focuses is therefore the development of automated programming of DBS electrodes. This will combine imaging techniques to analyze electrode positions in relation to surrounding anatomical structures and electrophysiological techniques to detect symptom-specific potentials in order to predict clinically effective stimulation *.
-
Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson’s disease and tremor. Mov Disord. 2006;21 Suppl 14:S259-83.
-
Hariz MI. Safety and risk of microelectrode recording in surgery for movement disorders. Stereotact Funct Neurosurg. 2002;78:146-157.
-
Kocabicak E, Alptekin O, Ackermans L et al. Is there still need for microelectrode recording now the subthalamic nucleus can be well visualized with high field and ultrahigh MR imaging? Front Integr Neurosci. 2015;9:46.
-
Nowacki A, Debove I, Fiechter M et al. Targeting Accuracy of the Subthalamic Nucleus in Deep Brain Stimulation Surgery: Comparison Between 3 T T2-Weighted Magnetic Resonance Imaging and Microelectrode Recording Results. Oper Neurosurg (Hagerstown). 2018;15:66-71.
-
Deuschl G, Schade-Brittinger C, Krack P et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896-908.
-
Kupsch A, Benecke R, Müller J et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978-1990.
-
Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66:289-296.
-
Pollo C, Kaelin-Lang A, Oertel MF et al. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain. 2014;137:2015-2026.
-
Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov Disord. 2016;31:1240-1243.
-
Akram H, Dayal V, Mahlknecht P et al. Connectivity derived thalamic segmentation in deep brain stimulation for tremor. Neuroimage Clin. 2018;18:130-142.
-
Coenen VA, Allert N, Paus S, Kronenbürger M, Urbach H, Mädler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery. 2014;75:657-69; discussion 669.
-
Nowacki A, Debove I, Rossi F et al. Targeting the posterior subthalamic area for essential tremor: proposal for MRI-based anatomical landmarks. J Neurosurg. 2018;131:820-827.
-
Nowacki A, Schlaier J, Debove I, Pollo C. Validation of diffusion tensor imaging tractography to visualize the dentatorubrothalamic tract for surgical planning. J Neurosurg. 2018;130:99-108.
-
Tinkhauser G, Pogosyan A, Debove I et al. Directional local field potentials: A tool to optimize deep brain stimulation. Mov Disord. 2018;33:159-164.
-
Holewijn RA, Verbaan D, van den Munckhof PM, Bot M, Geurtsen GJ, Dijk JM, Odekerken VJ, Beudel M, de Bie RMA, Schuurman PR. General Anesthesia vs Local Anesthesia in Microelectrode Recording-Guided Deep-Brain Stimulation for Parkinson Disease: The GALAXY Randomized Clinical Trial. JAMA Neurol. 2021 Oct 1;78(10):1212-1219.
