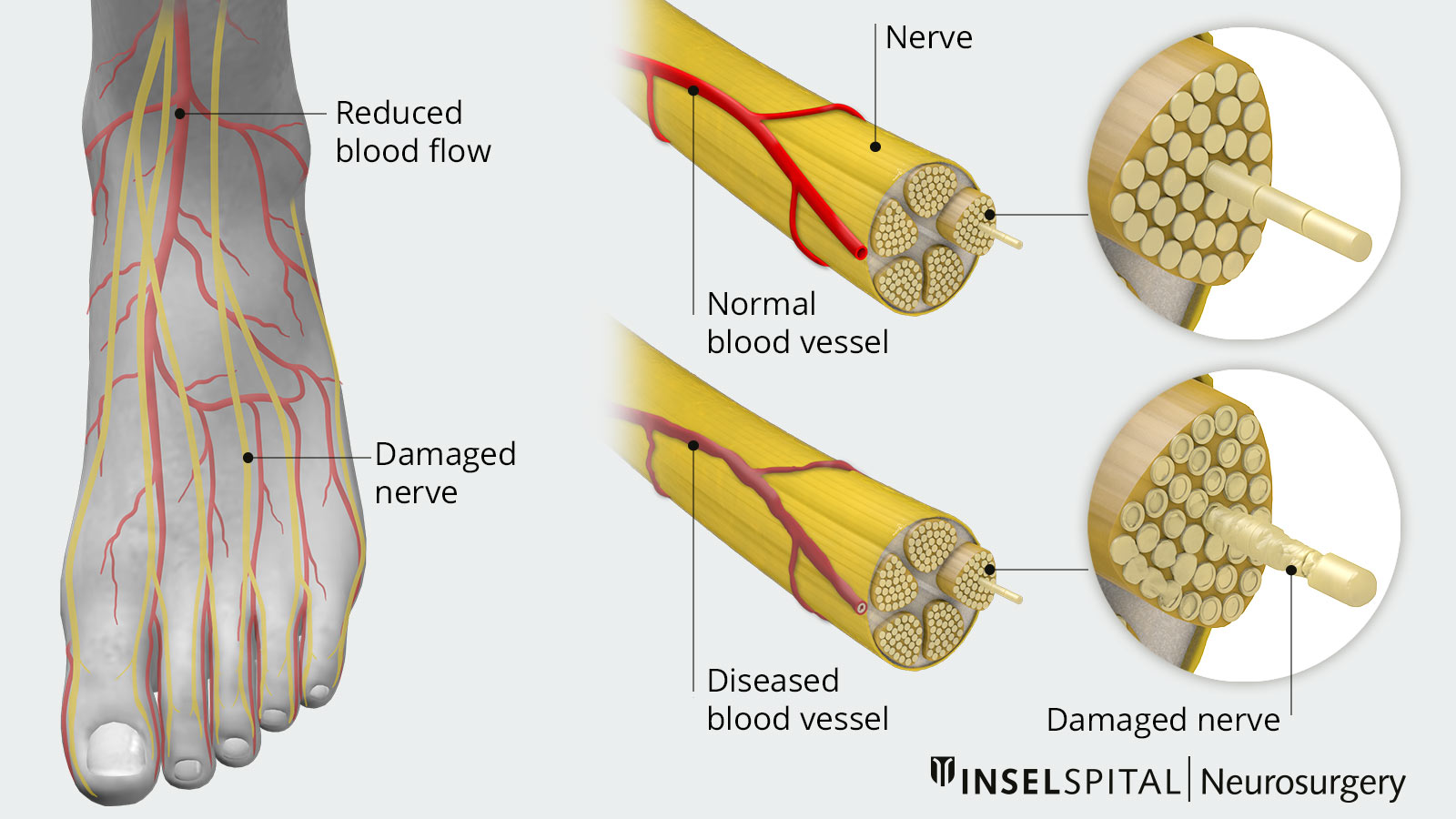
Diabetic neuropathy is a consequence of long-term diabetes mellitus. The excessively high blood glucose level damages the nerves. The long nerves that supply the legs are particularly affected. Affected patients suffer from sock-like sensory disturbances, gait unsteadiness and the feeling of walking on absorbent cotton. Approximately 20% of all patients with advanced diabetes mellitus also develop a neuropathic pain syndrome with burning pain in the feet and legs, which is extremely difficult to treat. Spinal cord stimulation can provide relief for affected patients and is offered as a surgical service at our Inselspital.
How does pain develop in diabetic neuropathy?
The constantly elevated blood glucose level in diabetes mellitus has negative effects on the entire organism. The most common long-term damage caused by diabetes mellitus affects the cardiovascular system (increased risk of heart attack and stroke) and the nervous system. In the latter, the long nerve tracts of the peripheral nervous system, which are responsible for sensory transmission and motor function in the lower extremities, are particularly affected. Chronic progressive nerve damage typically results in sensory disturbances in the feet and legs, which manifest as a dull feeling and are often also accompanied by burning, shooting or constricting pain.
What are the treatment options?
The only and most important causal therapy is the consistent adjustment of blood glucose levels to prevent the disease from progressing *. In addition, therapy of the symptoms is possible. Medications from the class of antiepileptic drugs, such as gabapentin (Neurentin) and pregabalin (Lyrica), and antidepressants, such as amitriptyline or duloxetine (Cymbalta), which are also effective and administered for other neuropathic pain, are primarily used to relieve pain *. Opioids are usually not very effective *. If these therapeutic attempts fail, neuromodulation in the form of spinal cord stimulation is an option.
Spinal cord stimulation for neuropathic pain
Suitable candidates for spinal cord stimulation are patients with chronic neuropathic pain due to diabetic neuropathy, whose blood glucose levels are well controlled, but in whom drug therapy is insufficiently effective and whose level of suffering is correspondingly high.
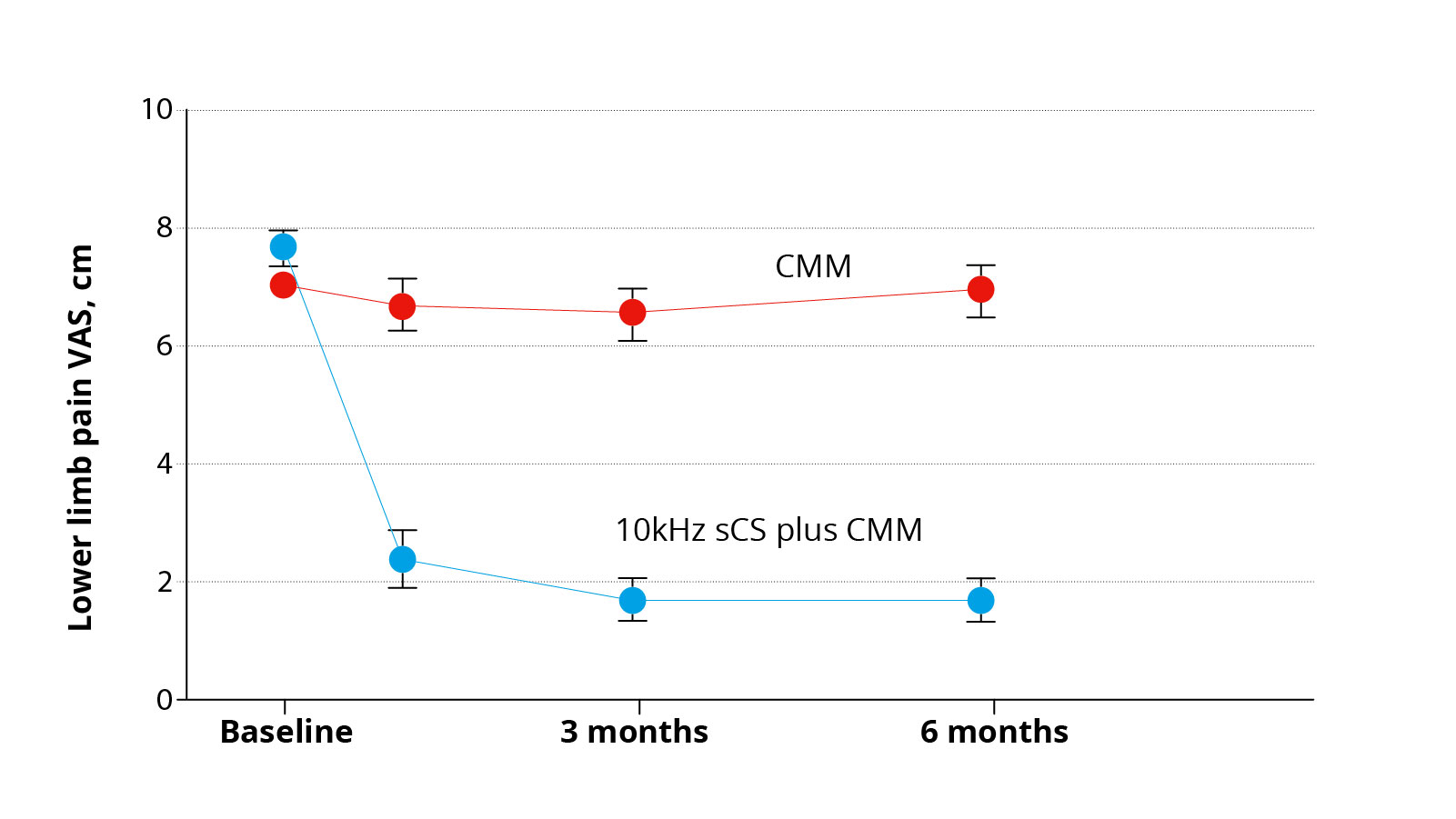
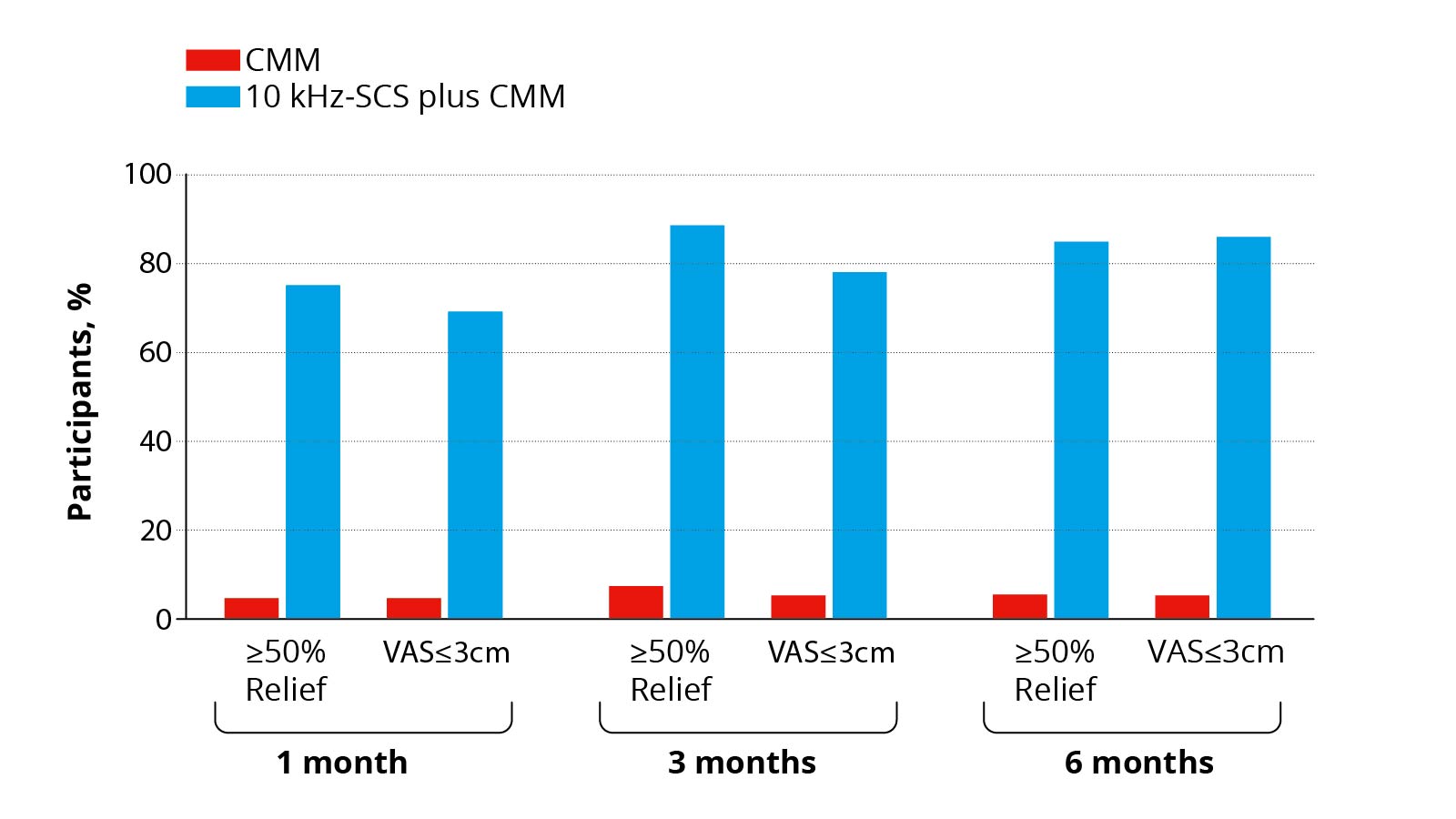
A recently published study in the journal JAMA Neurology demonstrates the efficacy of 10 kHz spinal cord stimulation for chronic neuropathic pain due to diabetic neuropathy *. In this study, affected patients were divided into two groups and randomized, i.e. assigned at random to a treatment group. Patients in the active group were tested for 10-KHz spinal cord stimulation in addition to drug therapy. If pain relief was satisfactory for the patient, the stimulator was implanted permanently. Patients from the control group were treated with medication alone. Both groups were observed over a period of 6 months and factors such as pain intensity, quality of life and neurological status of the patients were recorded and assessed. It was found that in the spinal cord stimulation group, 85% of patients benefited from at least 50% pain relief, while after drug-only therapy, only 5% of patients reported a 50% improvement in pain. On average, pain relief from spinal cord stimulation was approximately 75%. Even though the study does not record long-term results and the observation period of 6 months is relatively short, these results are very promising.
Our experience at Inselspital
Spinal cord stimulation is regularly performed successfully in our clinic. Potential patients with painful diabetic polyneuropathy are first thoroughly evaluated by a multidisciplinary team of neurologists, diabetologists and pain specialists. Good patient selection, taking into account many medical factors, is of enormous importance for the therapeutic success of spinal cord stimulation. Suitable patients first undergo a test phase of 1-2 weeks, during which the implanted electrode is connected to a mobile, external pacemaker device. If the patient benefits well from spinal cord stimulation, a second surgery is performed to connect the implanted electrode to a definitive pacemaker implanted under the skin in the subcutaneous fat tissue. If the test run has not resulted in sufficient pain relief, the implanted electrode can be withdrawn again in a short outpatient procedure.
-
Callaghan B, Little A, Feldman E, Hughes R. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database of Systematic Reviews. 2012;.
-
Waldfogel JM, Nesbit SA, Dy SM, Sharma R, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology 2017;88:1958-1967.
-
Bril V, England J, Franklin GM, Backonja M, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011;76:1758-1765.
-
Petersen EA, Stauss TG, Scowcroft JA, Brooks ES, et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA neurology 2021;78:687-698.