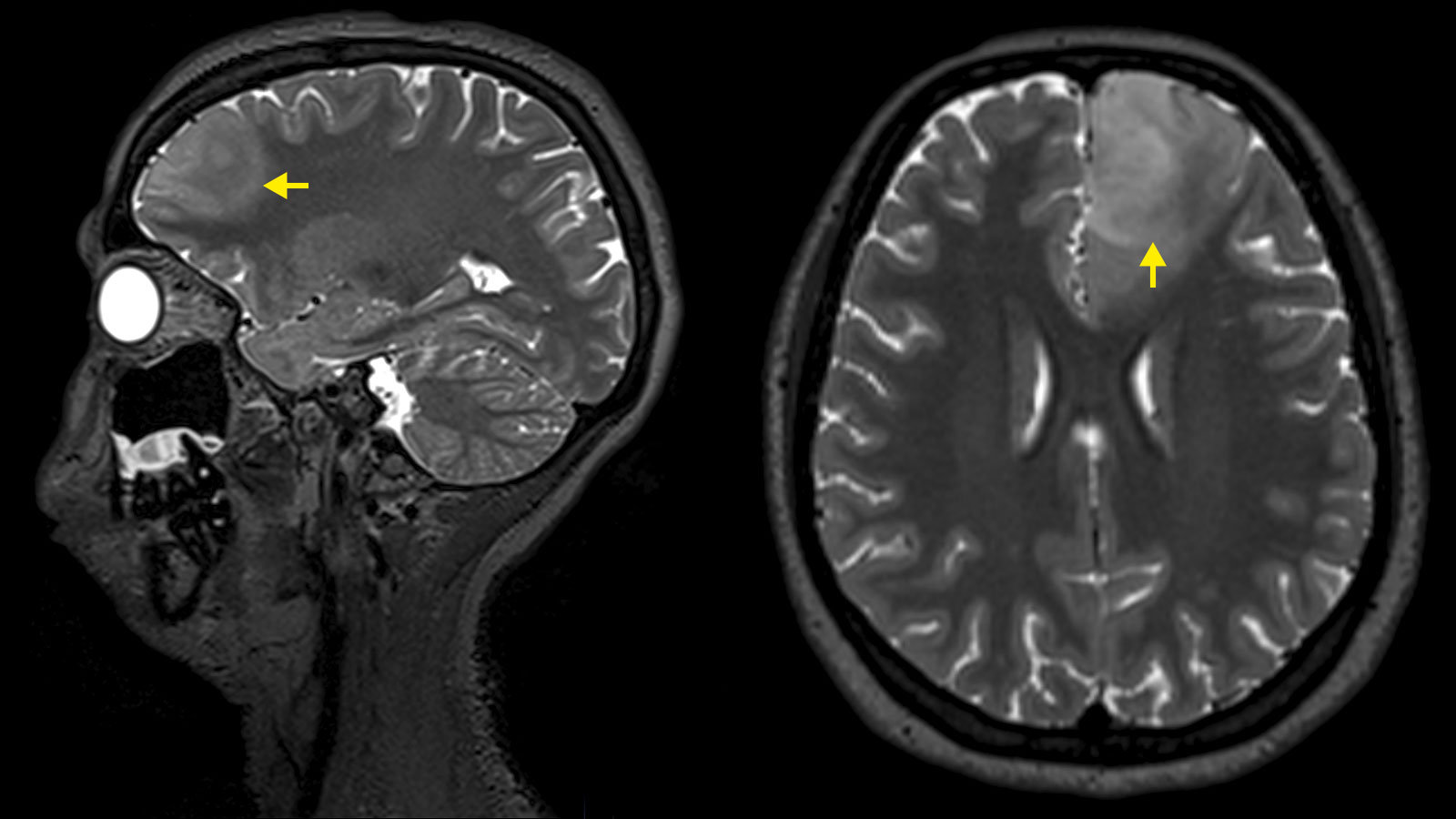
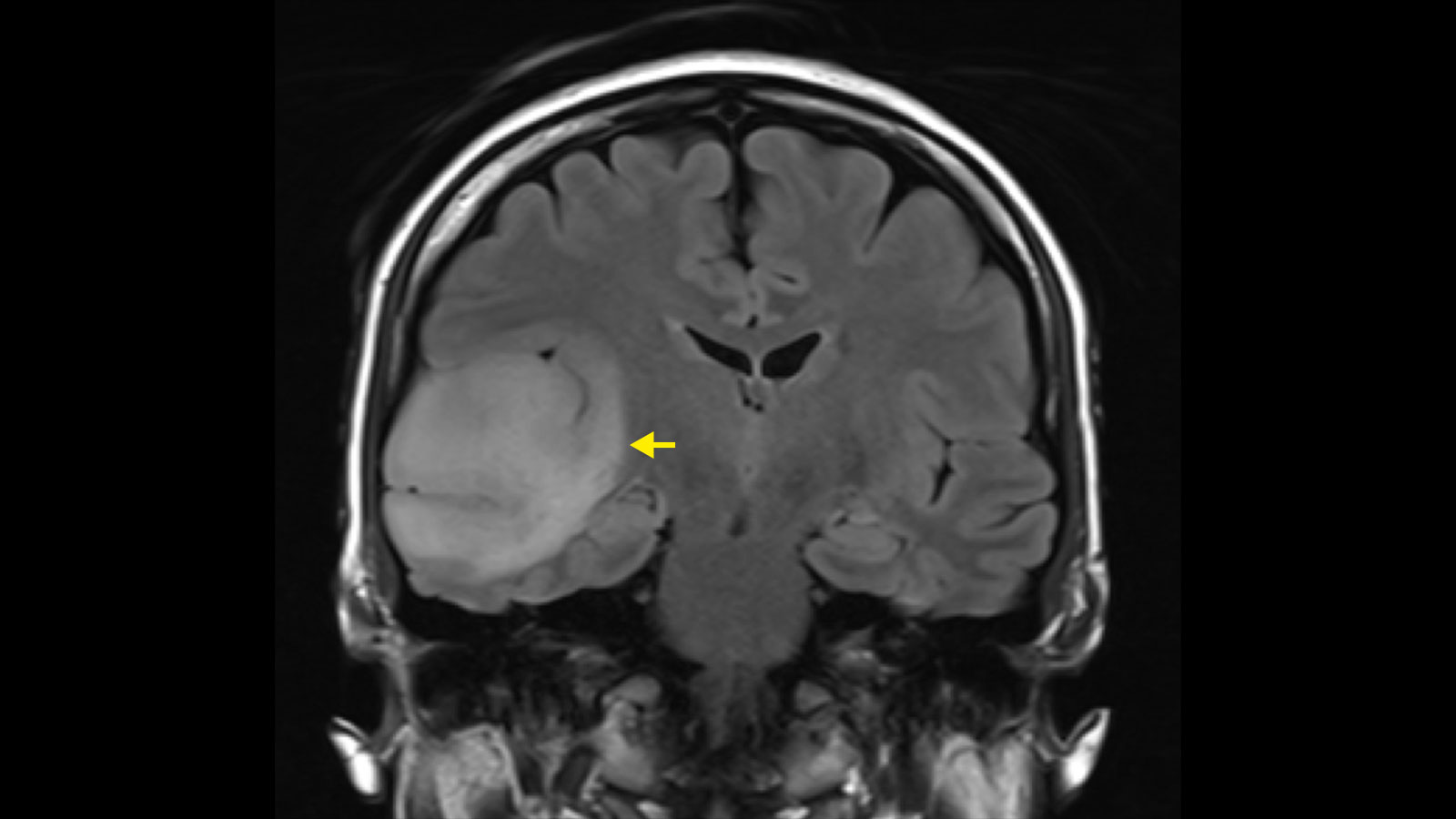
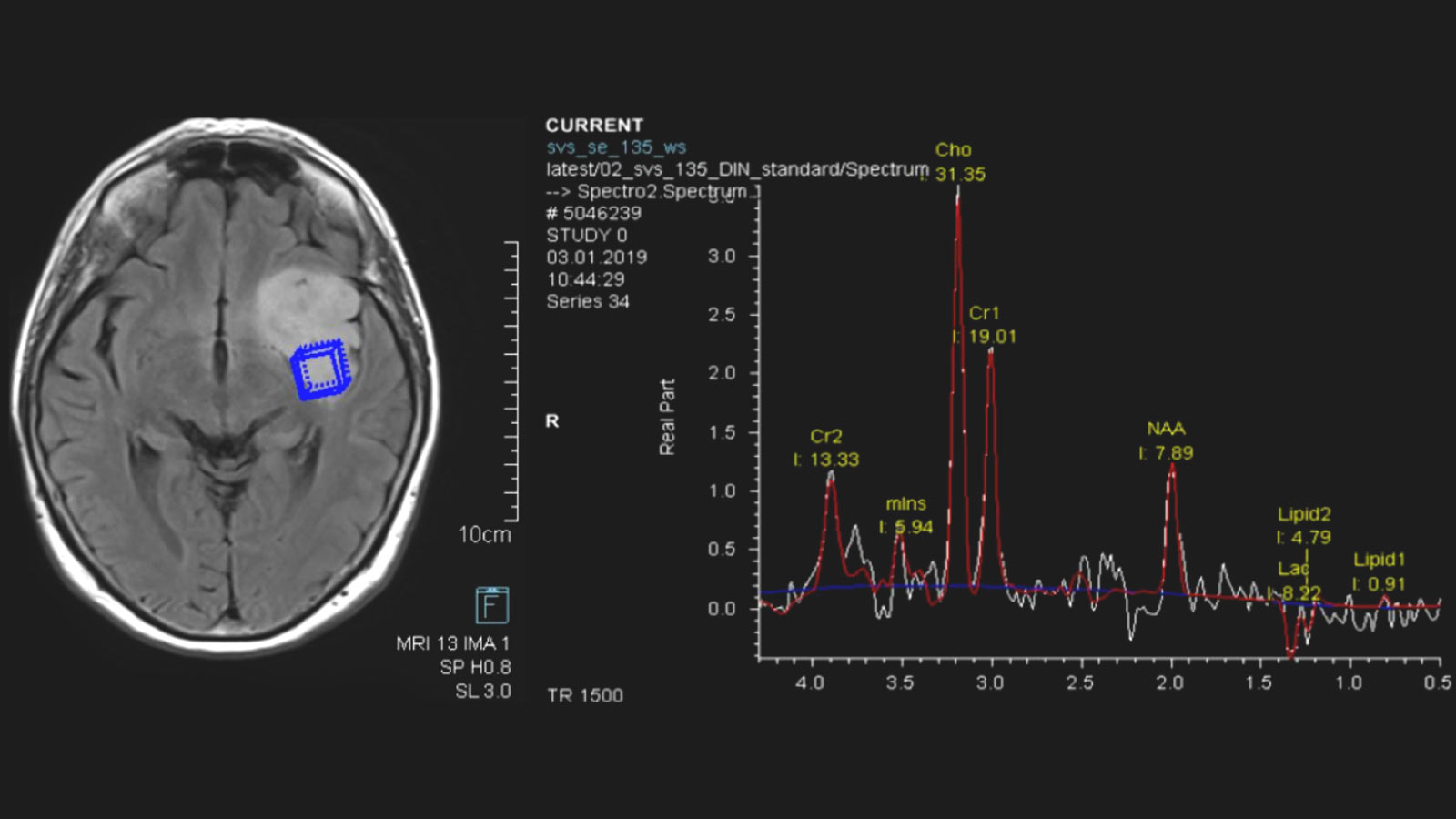
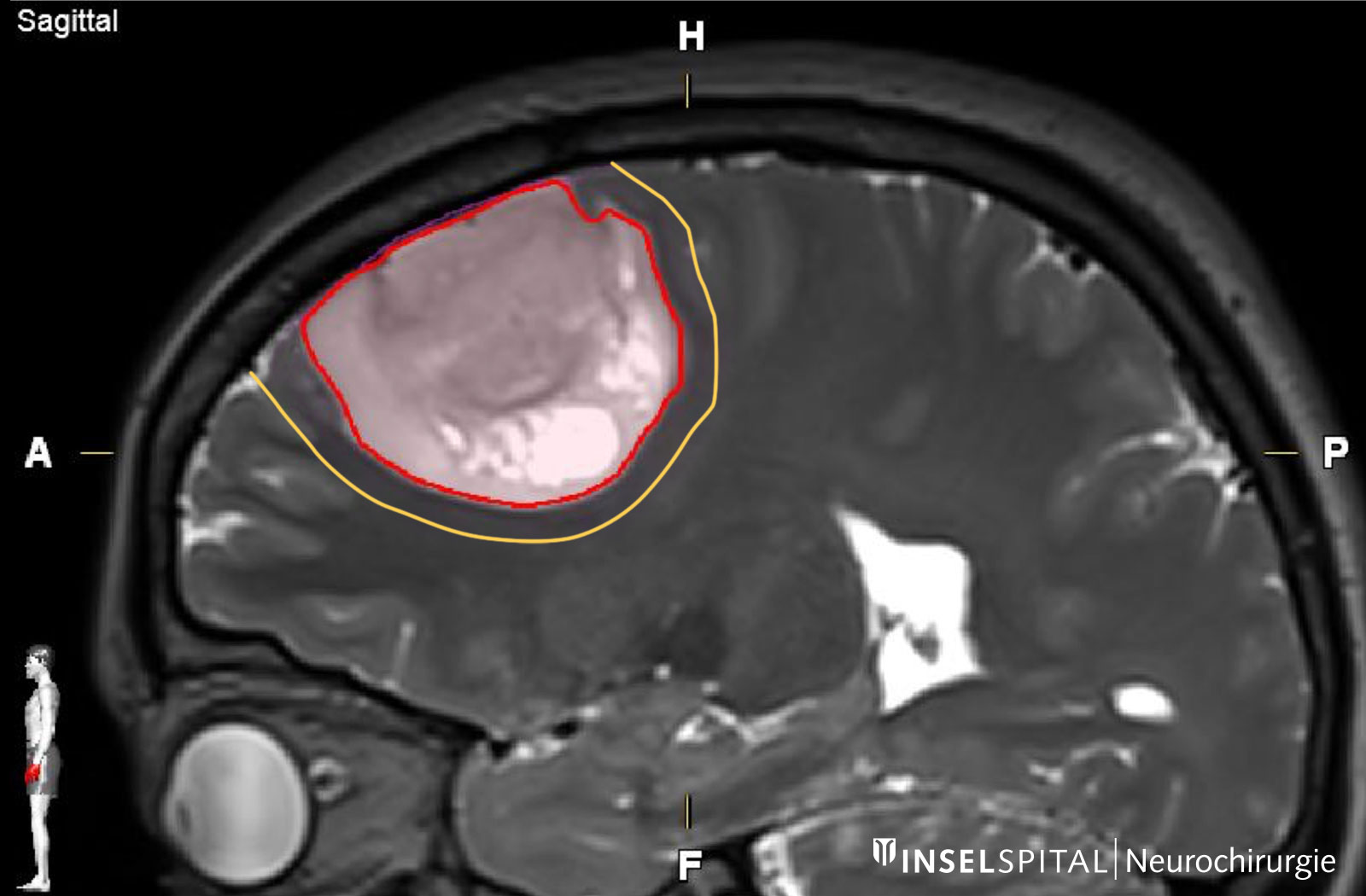
Astrocytomas and oligodendrogliomas are low-grade brain tumors from the glioma group. They tend to grow slowly – over years or even decades. However, there is a risk that a low-grade glioma will undergo malignant transformation and then continue to grow aggressively. This transformation depends on the tissue type of the tumor, its molecular characteristics and its size. The standard therapy for astrocytomas and oligodendrogliomas is surgery – supplemented, if necessary, by chemotherapy and radiation.
Inselspital, Department of Neurosurgery – our facts and figures
- Excellence: highly specialized neurosurgeons and specially trained oncology nurses (Advanced Practice Nurses or APNs for short).
- Expertise: 447 tumor surgeries (biopsies and resections) in 2023, of which 322 were gliomas
- Interdisciplinary team: specialists from 7 specialties meet in our weekly tumor board – neurosurgery, neurology, neuroradiology, oncology, nuclear medicine, radiation oncology, pathology
- Developed and researched at Inselspital: innovative neuromonitoring and navigation techniques for surgical safety and avoidance of deficits
- Deficit rate among the lowest worldwide: documented and published rate of 3–5% for permanent surgery-related deficits in motor eloquent risk tumors is among the lowest worldwide!
- Resection rate among the highest worldwide: The rate of > 90% complete resection for gliomas is among the highest worldwide!
- State-of-the-art technological equipment with intraoperative imaging, fluorescence techniques, laser thermotherapy and more.
- Complementary treatment concept: our OPTIMISST protocol (OPTIMISST stands for Optimized Standard and Supportive Therapy).
- Certification: Certified Brain Tumor Center since 2016, guaranteeing a high quality standard in oncological treatment.
Classification
Astrocytomas and oligodendrogliomas are among the most common low-grade gliomas and typically have an IDH mutation. The term "low-grade" actually corresponds to a grade 2 according to the WHO classification of central nervous system tumors. However, physicians cannot confidently assign a glioma to a grade 2 or a grade 3 based on either the MRI image or the tissue classification. Therefore, the growth, recurrence, and degeneration propensity of such a tumor cannot be reliably predicted. Today, we speak of low-grade gliomas as distinct from aggressive glioblastomas (grade 4) or IDH wild-type tumors.
How common are low-grade gliomas and who is affected?
Generally, these are rare tumors with an annual new diagnosis rate of 1 per 100,000 population *.
Astrocytomas and oligodendrogliomas typically occur in young adults with an average age of 35–45 years * * *.
The only known clear risk factor for the development of low-grade glioma is previous irradiation of the head.
Hereditary factors play only a minor role. However, these tumors occur more frequently in patients with neurofibromatosis type 1 or Li-Fraumeni syndrome, two rare hereditary diseases.
What is the cause of low-grade gliomas?
The exact cause for the development of astrocytomas and oligodendrogliomas is unclear. Nowadays we know many mutations of the DNA of tumor cells, which are typical for low-grade gliomas and give us important clues about the behavior of the tumor. However, these mutations are rather markers than triggers.
- Genetic markers
As mentioned in the classification, a defining feature of typical low-grade gliomas is the presence of an IDH mutation.
Additional genetic mutations then further subdivide these tumors into astrocytomas and oligodendrogliomas.
Oligodendrogliomas are said to occur when there is a specific alteration of two chromosomes, namely a loss of the short arm of chromosome 1 and the long arm of chromosome 19, a so-called unbalanced translocation (loss of heterozygosity or co-deletion1p/19q).
In astrocytomas, this codeletion 1p/19q is not present, but a mutation in the ATRX gene is typically found here.
- The p53 gene
Another important role is played by the p53 gene, whose product acts as a tumor suppressor and is therefore an important control in the regulation of cell growth. Mutations in p53 are detected in a large number of low-grade gliomas, especially when transformation to a higher-grade glioma occurs.
Early in the developmental phase of a low-grade glioma, an alteration is observed in the MDM2 gene, which acts as a regulator of p53 and thus has the same genetic mechanism of action.
What are the typical symptoms?
Low-grade gliomas grow slowly and only slightly disrupt the normal functions of the affected brain area. The slow growth allows the brain to adapt and shift important functions from the tumor area to other, healthy areas of the brain. This functional adaptation is called plasticity. Astrocytomas and oligodendrogliomas can thus grow to a considerable size over years without causing any symptoms. It is not uncommon for these tumors to be discovered quite by accident on MRI after imaging has been ordered for some other reason.
In 80% of cases, an epileptic seizure is the first symptom.
Other nonspecific symptoms such as headache, change in demeanor, fatigue, dizziness, unsteadiness of gait, nausea, and vomiting are possible but usually do not occur until the tumor has reached a large volume. Between epileptic seizures, most patients are symptom-free.
How are low-grade gliomas diagnosed?
Magnetic resonance imaging
Diagnosis is primarily made by magnetic resonance imaging (MRI). On the MRI image, astrocytomas and oligodendrogliomas show up as bright areas in the T2-weighted MRI sequences. Although astrocytomas and oligodendrogliomas can be clearly delineated on MRI, tumor cell nests can be found up to at least 2 cm around the visible tumor and single tumor cells can even be found beyond that * *. Therefore, astrocytomas and oligodendrogliomas are infiltrating brain tumors * *.
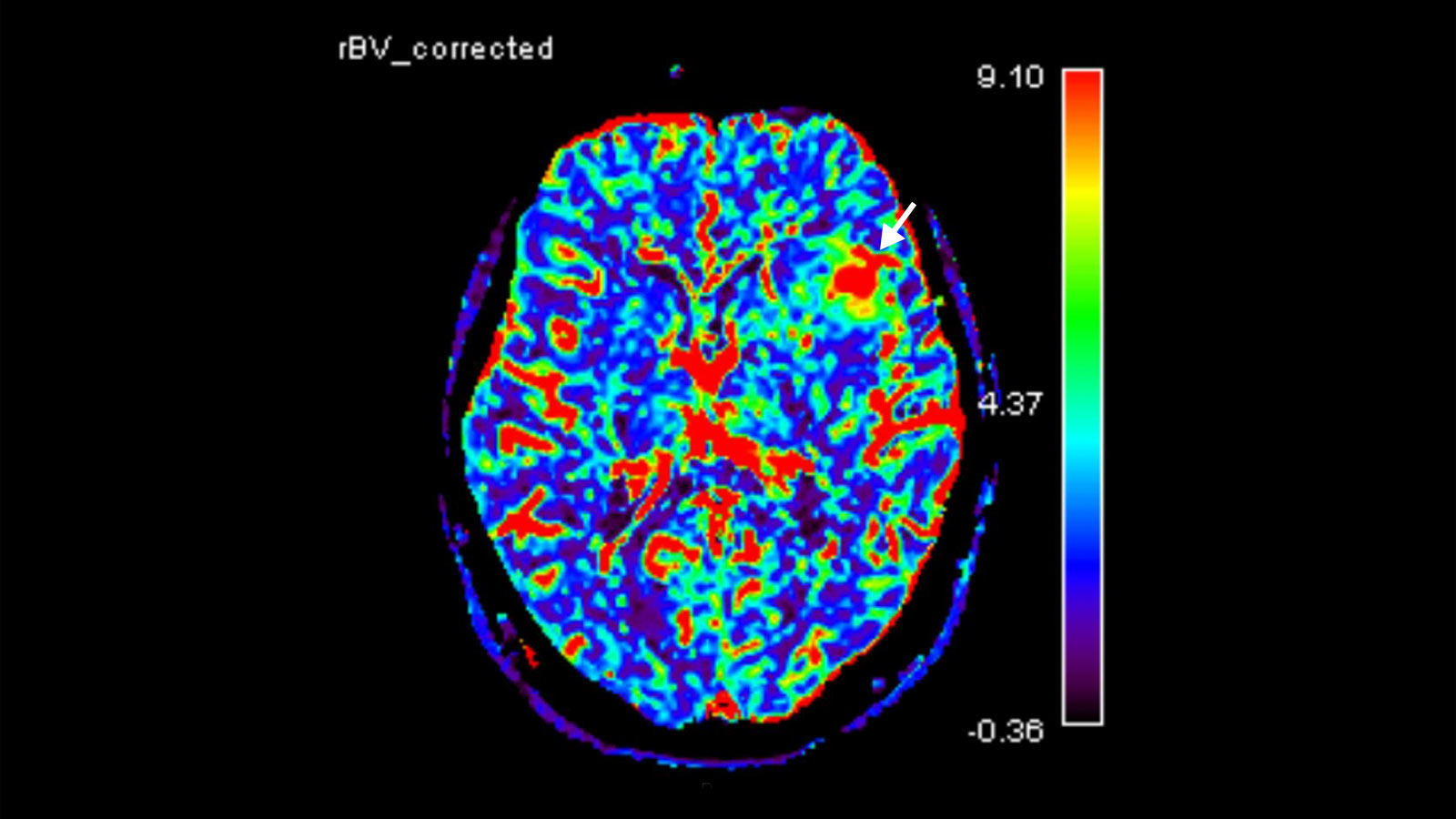
Neuroimaging
Further imaging differentiation can be achieved by means of "advanced neuroimaging". This includes methods for measuring local blood flow (perfusion) and local metabolites using magnetic resonance spectroscopy (MR spectroscopy or MRS).
Biopsy and tissue analysis
However, the suspected diagnosis after an MRI examination must be confirmed by tissue analysis after tumor removal or using a biopsy.
How are low-grade gliomas treated?
Malignant transformation
Since low-grade gliomas grow very slowly, no significant change in the tumor can be expected within a few months. For this reason, surgery was often delayed for a long time in the past. However, as is now known, this long wait means a higher risk of malignant transformation, i.e. degeneration of an astrocytoma or oligodendroglioma into a malignant tumor.
Although sometimes referred to as such, low-grade gliomas are not truly benign tumors. They grow slowly but steadily at an average rate of 0.5 to 4 mm/year * *. Spontaneous cures without therapy do not occur.
Malignant transformation determines prognosis and life expectancy in most patients. Unfortunately, the timing of this cannot be predicted. In the scientific literature, the risk is estimated at 3–10% per year, depending on histology, molecular characteristics and tumor size. Thus, the risk increases by about 10% per cm of tumor size.
However, it is now also known that complete tumor removal of the tumor visible on MRI can significantly reduce the risk of malignant transformation.
What factors determine the long-term success of the treatment?
Factors that can be influenced
- Time to surgery
The longer the waiting time in years, the higher the rate of malignant transformation.
- Extent of tumor resection
Standard goal is reduction, but the optimal goal is complete removal with additional margin (supramarginal removal) for the best prognosis after surgery.
- Therapy
Implementation of chemotherapy for inoperable tumors or tumor remnants, in combination with radiotherapy if necessary.
Factors that cannot be influenced
- age of the patient
- initial size of the glioma
- pre-existing neurological deficits due to the glioma
- genetic molecular profile of the glioma
- tissue type
- multiple tumor localizations
Our OPTIMISST protocol
There are a number of factors that contribute to tumor treatment being more successful. We have developed our own treatment concept based on these factors, the so-called OPTIMISST protocol. OPTIMISST stands for "Optimized Standard and Supportive Therapy" and complements the classic therapy for brain tumors. This protocol exists in this form only at our clinic.
Surgery – the standard therapy
The standard therapy for astrocytomas and oligodendrogliomas is surgical removal or resection * * * * * *.
There are 3 levels of radicality for surgery:
- a reduction of the tumor mass
- a complete removal of the tumor visible in the MRI image
- a complete removal of the tumor visible in the MRI image with a safety zone (supramarginal resection)
The more of the tumor can be removed during surgery, the more favorable the further course of the disease: The disease progresses more slowly, the time to malignant degeneration is delayed and the survival time is prolonged * * * * * * * * * * * * * *.
The aim of resection is therefore to radically remove the tumor up to the border of the functioning brain tissue. Since the distinction between tumor and brain tissue is often difficult, especially in the marginal area, various modern technologies are used here.
Surgery with margin: the supramarginal resection
Astrocytomas and oligodendrogliomas have tumor cells that extend beyond the margin visible on MRI. With supramarginal resection, an additional safety margin of 1–2 cm is also removed, which has a positive influence on the probability of recurrence * * *.
During surgery, the tumor is removed with this safety margin, provided that neuromonitoring does not detect any important brain functions there. This technique should always be performed simultaneously with neurophysiological monitoring and mapping to avoid permanent neurological damage.
Surgical safety
- developed at Inselspital: dynamic mapping and low-threshold mapping
- Inselspital leads the field in surgical safety through state-of-the-art mapping methods
- less paralysis compared to previous methods
- more radical tumor removal possible even in the immediate vicinity of motor center and power pathways
Magnetic resonance imaging
For optimal preparation, a special MRI is first performed. This allows the tumor to be assessed as accurately as possible and the operation to be planned precisely. Depending on the localization of the tumor, further examinations may be necessary.
A functional MRI (fMRI) can contribute valuable information on the localization of speech function and the motor center.
Navigated transcranial magnetic stimulation
Navigated transcranial magnetic stimulation (nTMS) is often performed for the precise localization of the centers of motor activity. This is a non-invasive examination that is tolerated by most patients without any problems and is offered at Inselspital as part of a multicenter study. The data collected in this way help the surgeon to plan the surgical approach and to determine the surgical strategy.
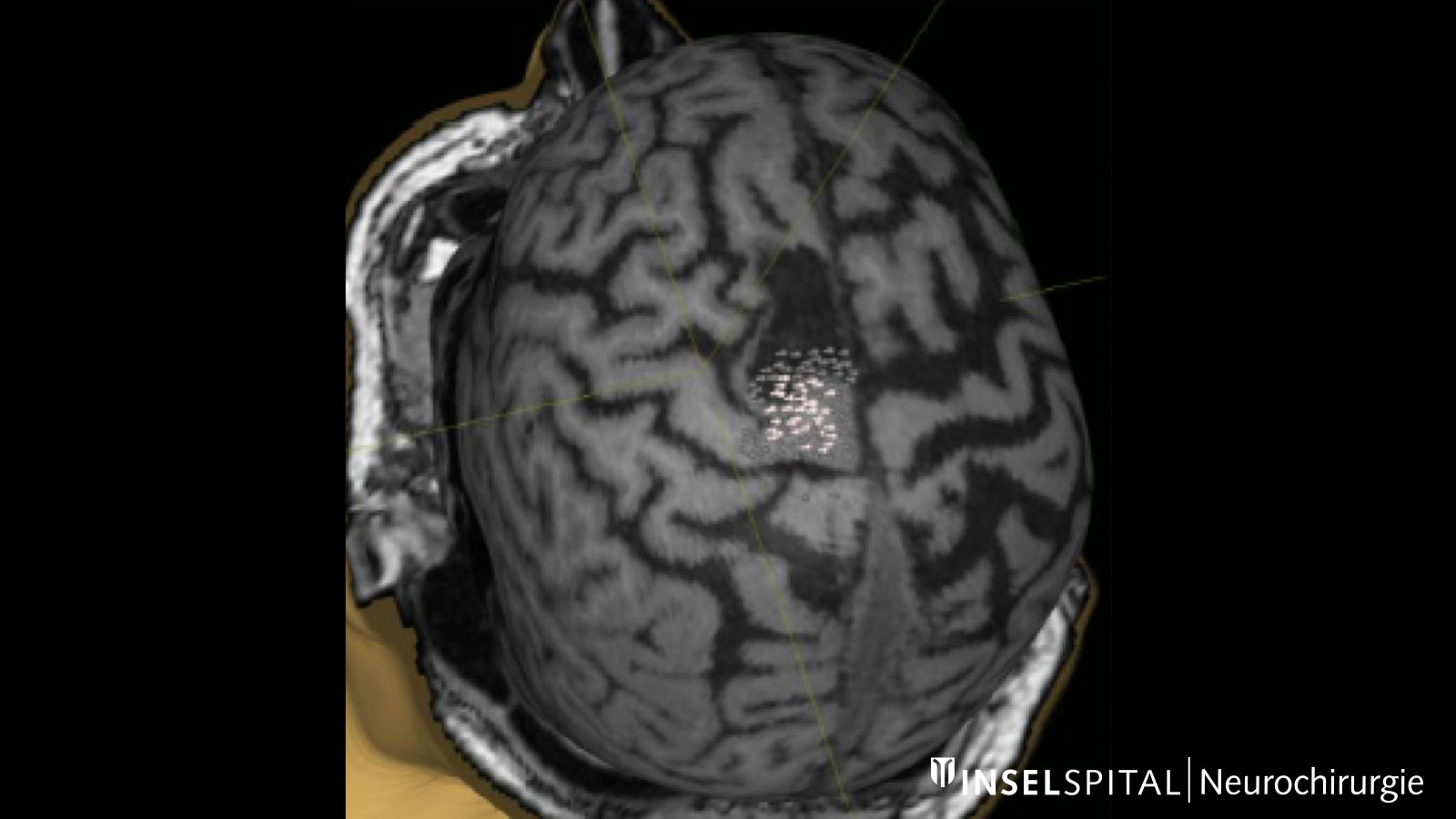
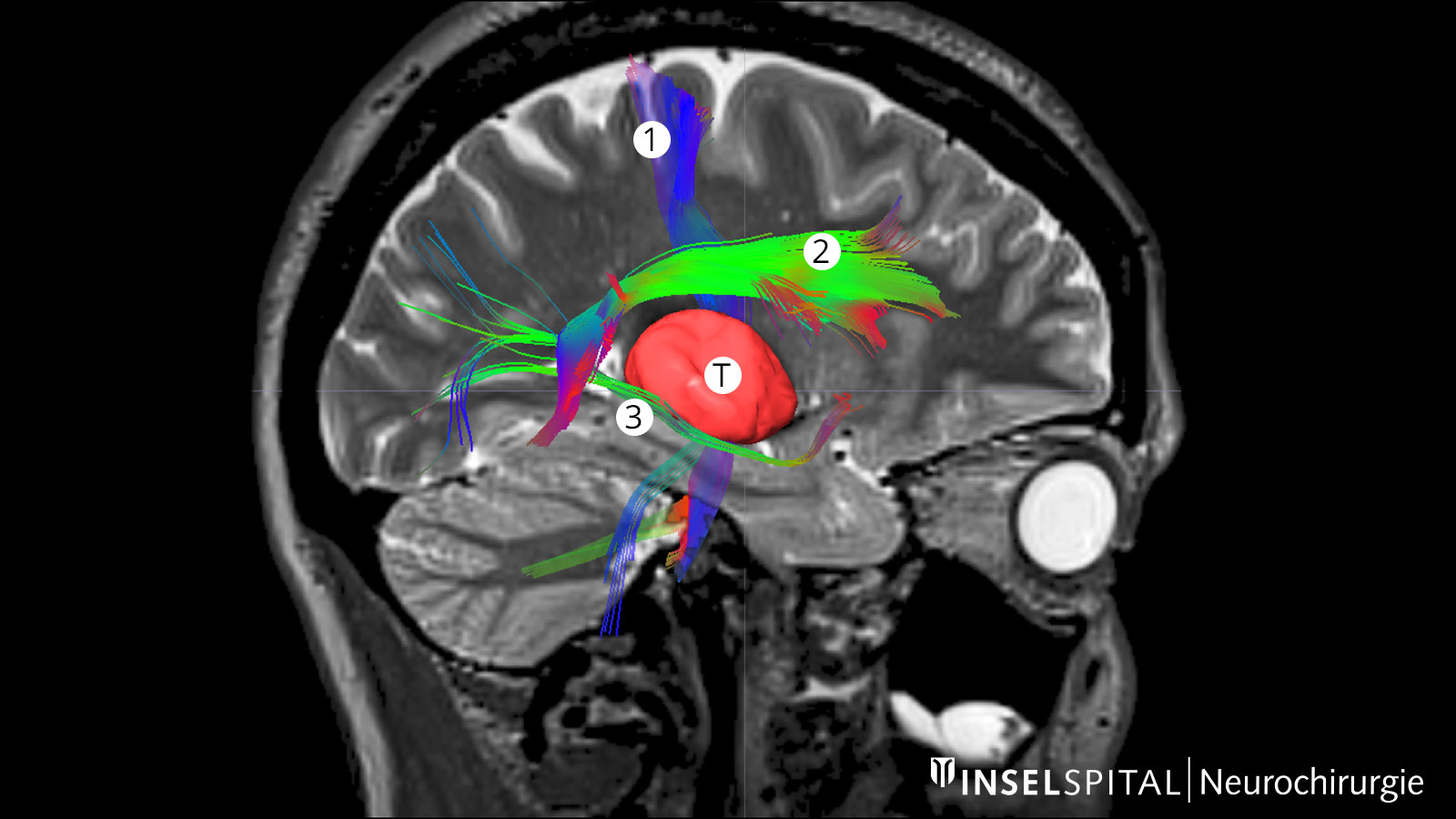
Fiber tracking: visualization of the brain fiber tracts
Tractography or fiber tracking are procedures used to visualize the fiber tracts in the brain. Visualizing these tracts before surgery helps the surgeon to find the optimal and safest path to the tumor past all important fiber tracts.
This is extremely important for the pyramidal tract. It represents the direct connection between the motor center of the brain and the spinal cord and must be spared at all costs during surgery. Other important fiber tracts primarily concern brain functions such as speech and vision.
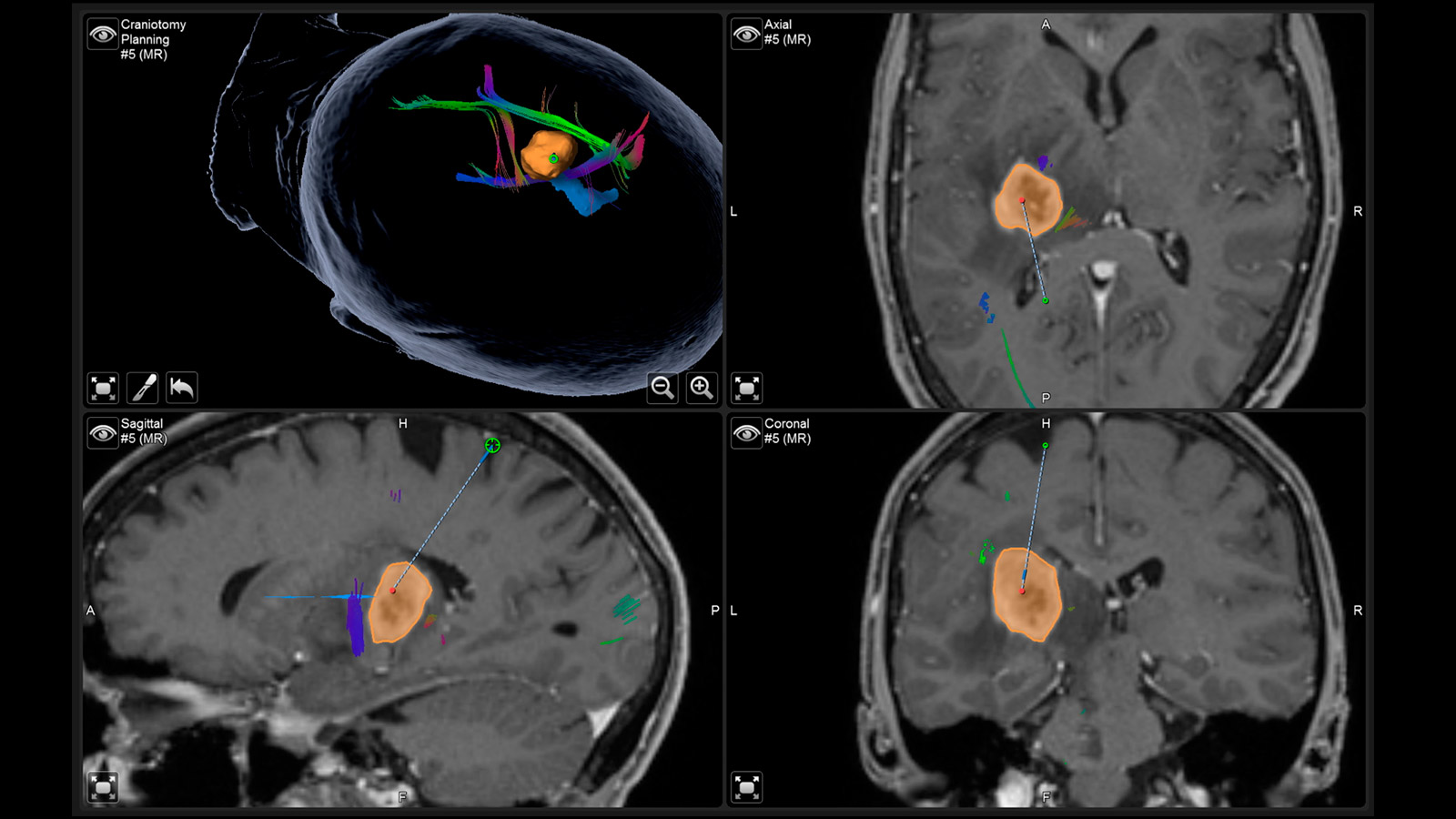
Neuronavigation
Neuronavigation works like a GPS during surgery and shows the neurosurgeon the way, with the MRI performed before surgery providing the individual "map material"
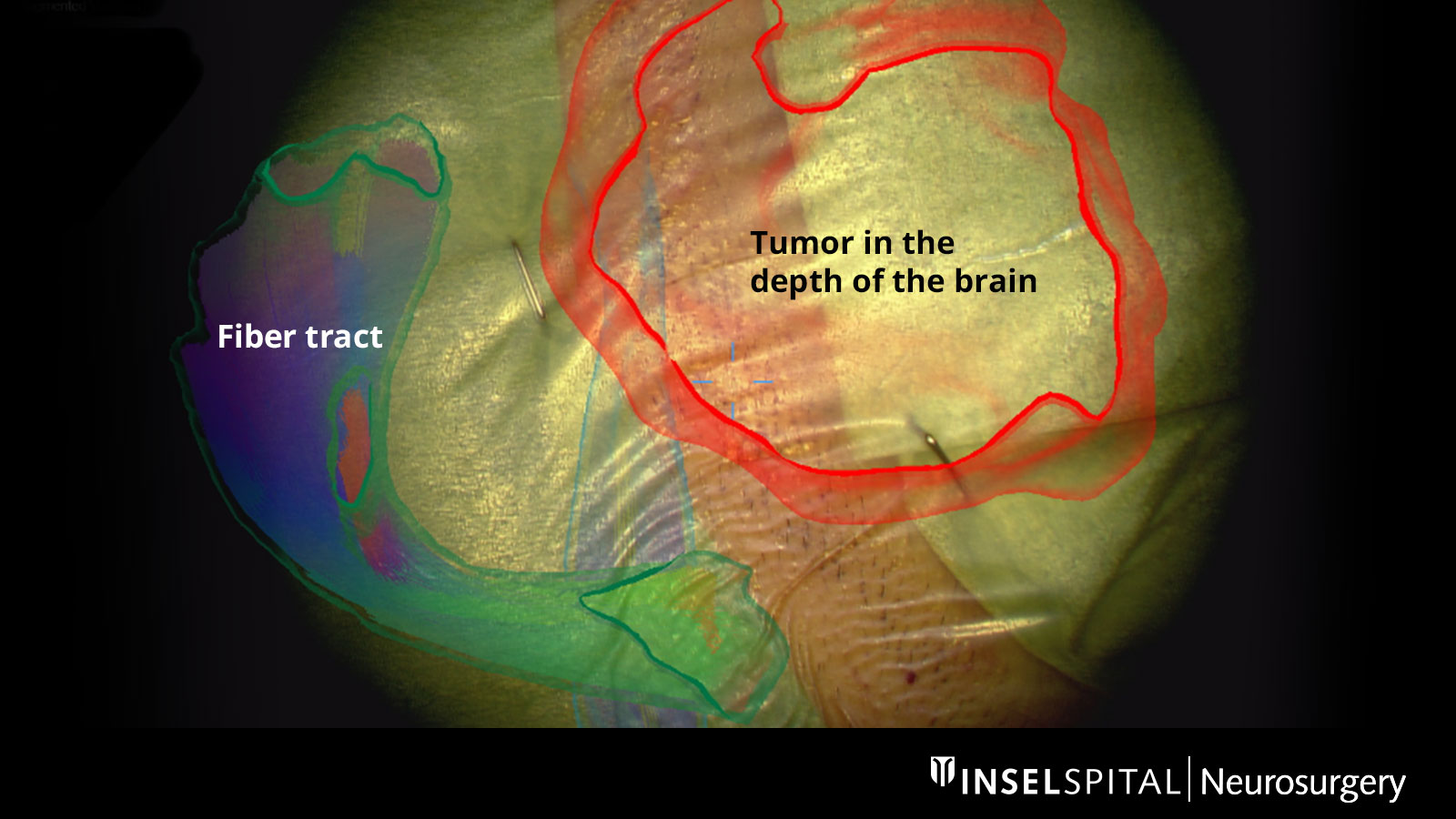
Augmented Reality
The fiber tracts already drawn in before the operation, as well as the tumor itself and other important areas, can be virtually superimposed in the operating microscope and projected onto the surface of the head. With the help of this augmented reality, the surgeon can orient himself better and plan the access to the tumor as small as possible, but as large as necessary.
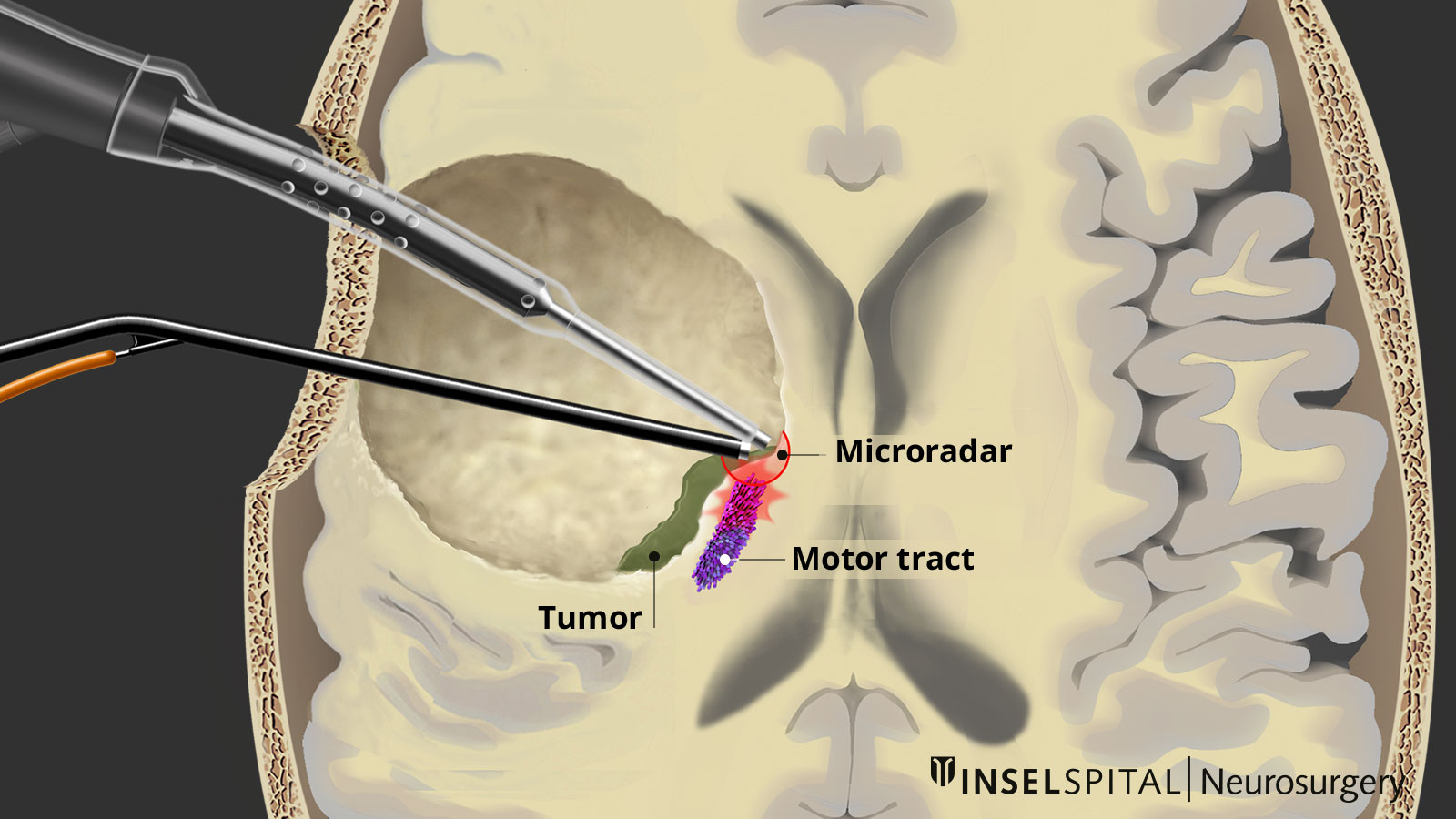
Dynamic mapping
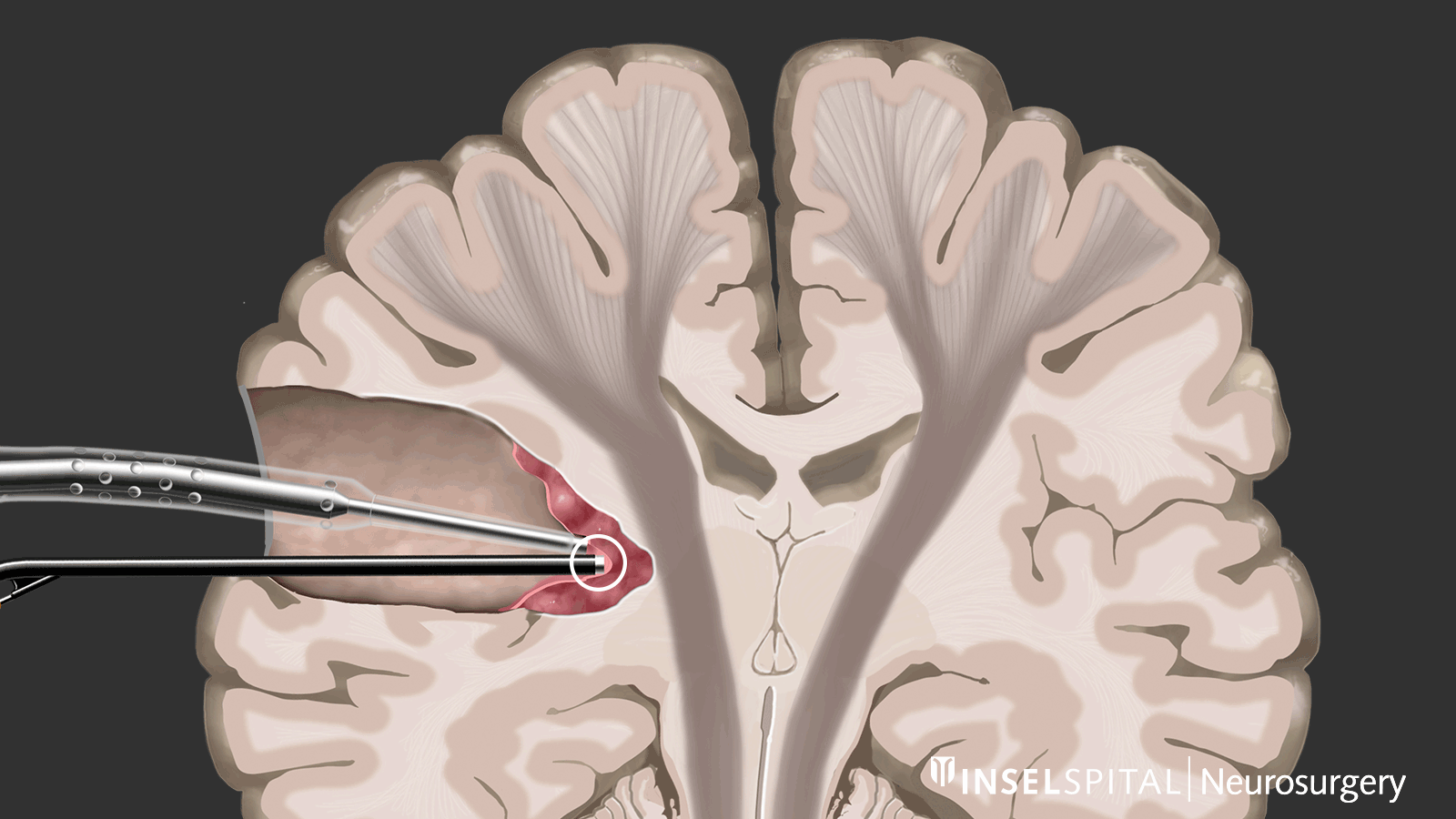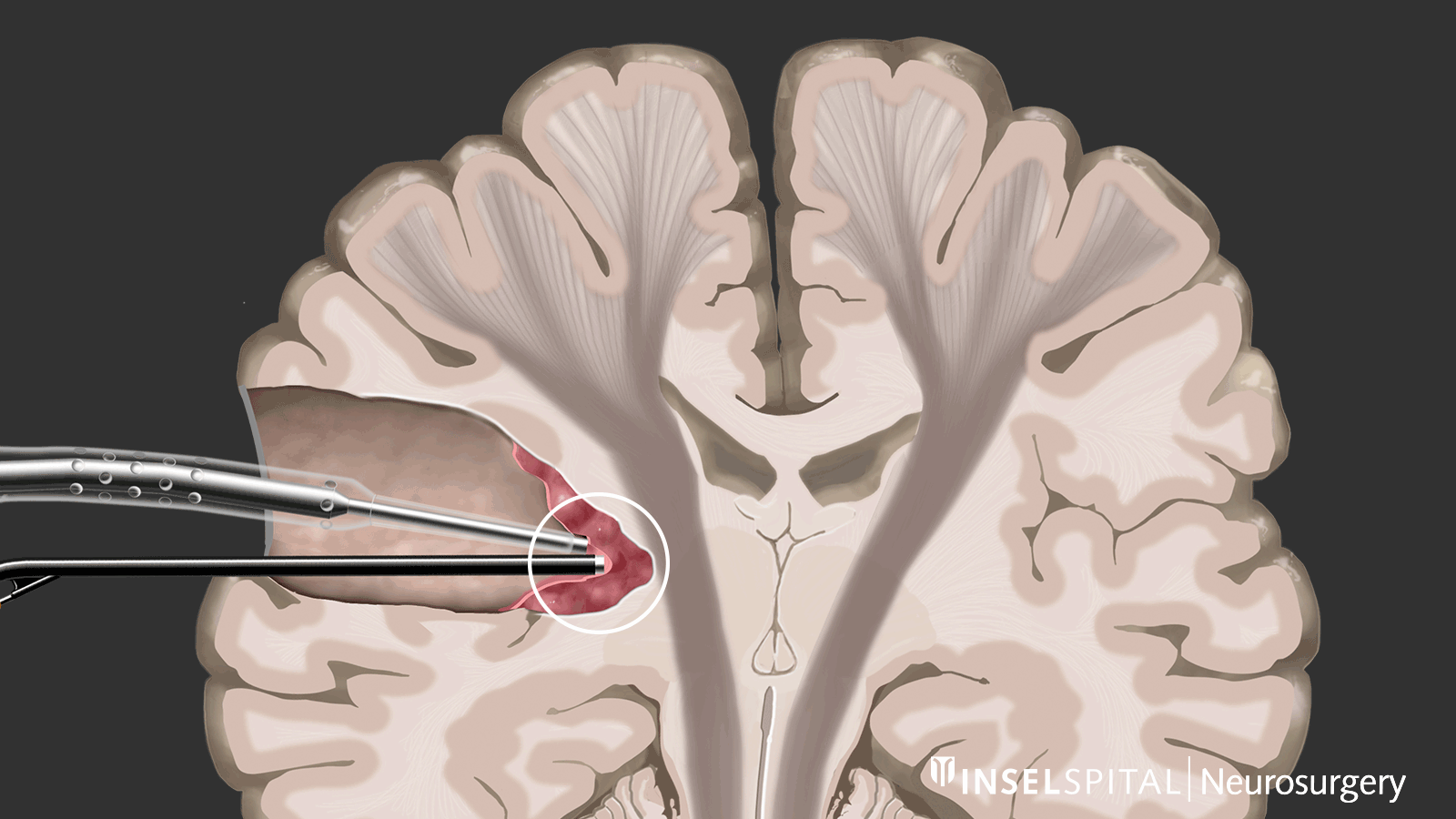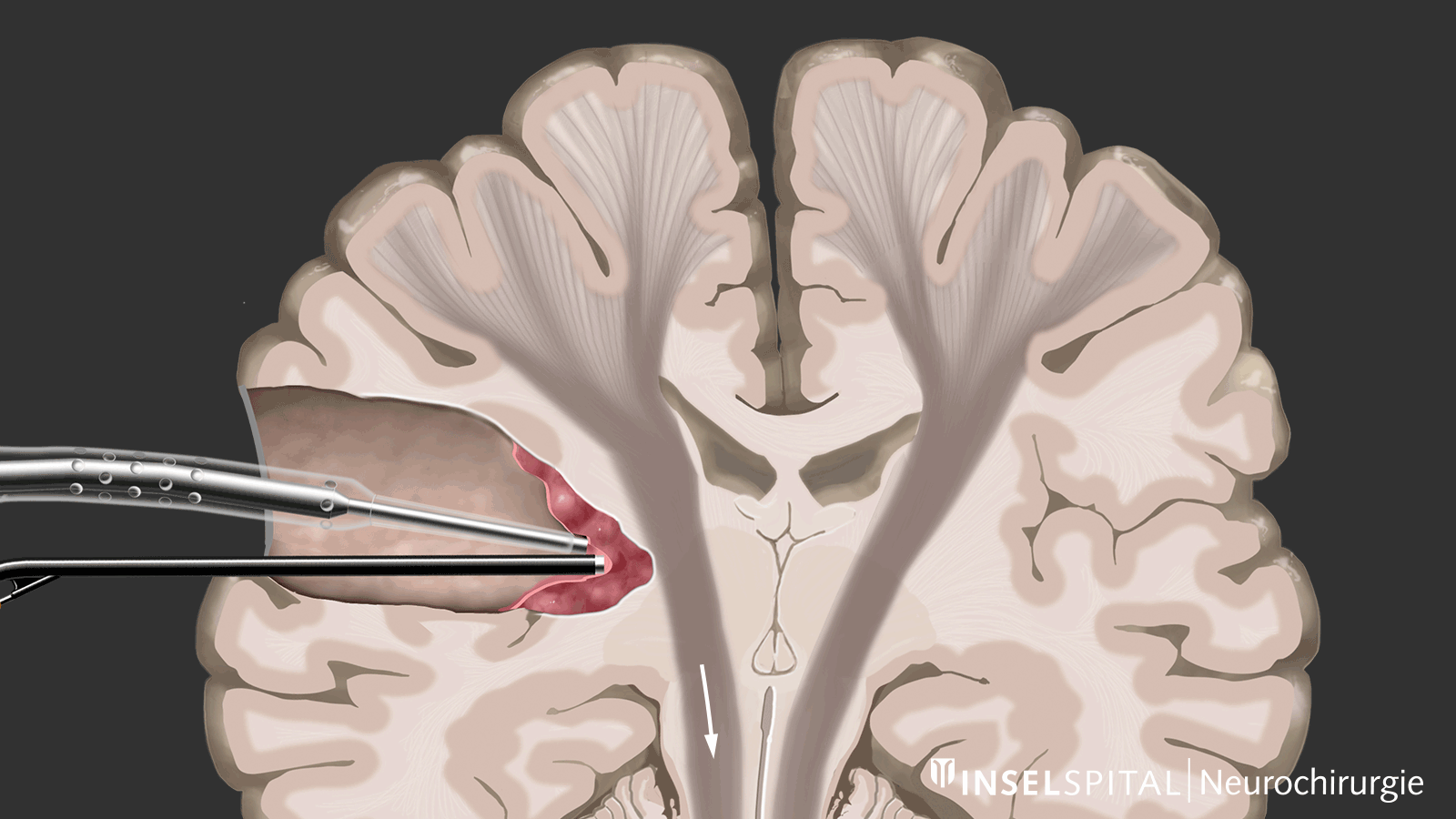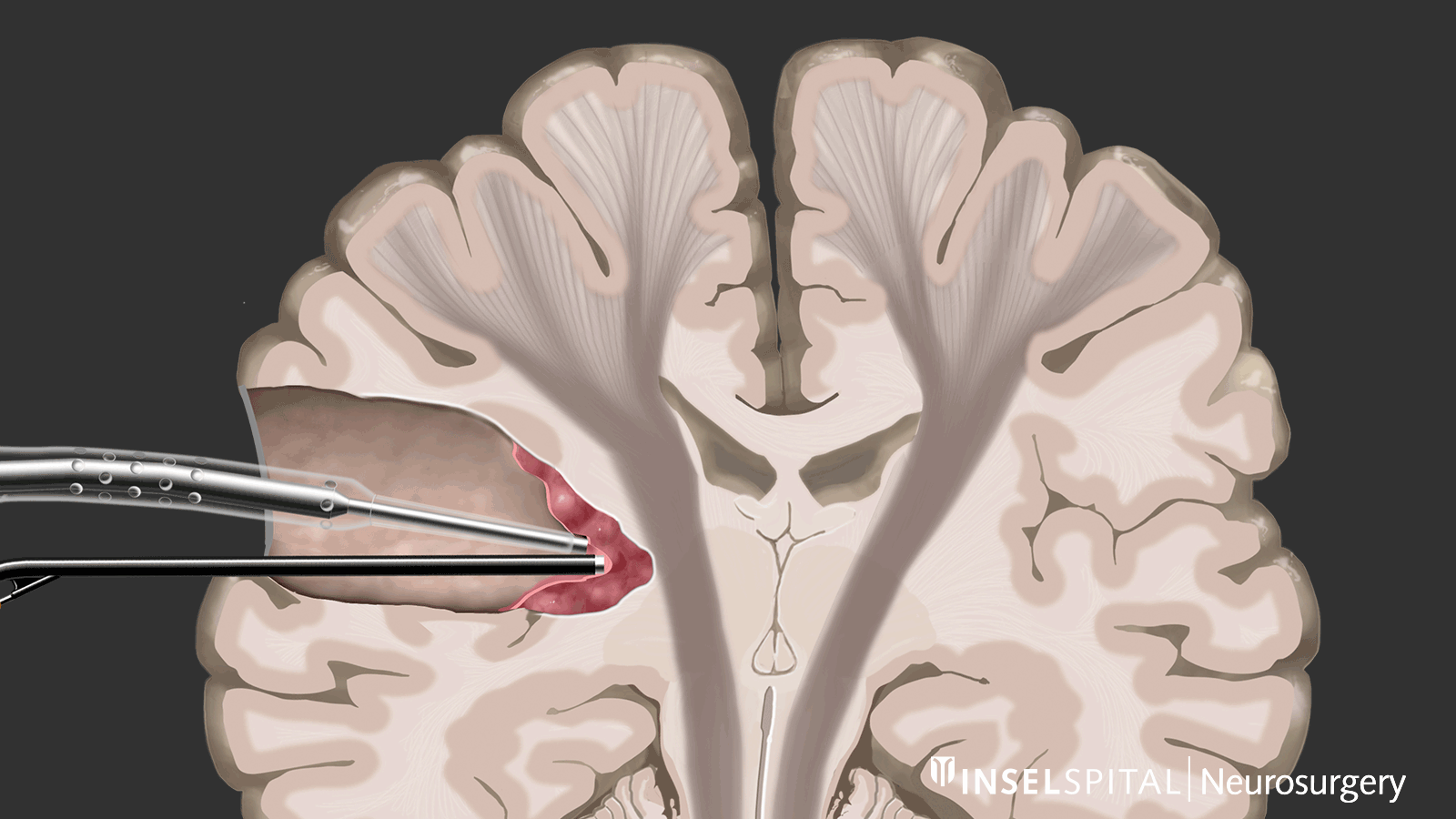
Dynamic mapping is the continuous scanning of brain tissue at the site of tumor removal with a radar of microcurrents. Surgery for glioma should always be performed with specialized functional mapping of speech, movement, vision, and higher brain functions when the tumor location is critical.
Thanks to this procedure and comprehensive intraoperative monitoring, the complication rate for such operations is nowadays very low * * * * * * * *.
Dynamic mapping
Awake surgery
Unlike motor functions, complex functions such as speech, language comprehension, arithmetic, music-making or reading cannot be monitored in patients under anesthesia. Therefore, it is necessary that the patient is awake during part of the surgery and performs special tests together with a neuropsychologist. The location of an invisible brain function can thus be localized and spared during surgery.
Awake surgery
Intraoperative imaging
Since the distinction between healthy tissue and tumor tissue is difficult even under the microscope, various imaging modalities are used during surgery to directly control the best extent of resection.
Ultrasound is routinely used but has its limitations in visualizing residual tumor tissue.
The most accurate method is intraoperative MRI. For this purpose, after removal of the tumor, the patient is transferred to MRI while the operation is still in progress. In this way, a tumor remnant, if present, can still be removed during the ongoing operation. For this purpose, an operating room with direct connection to a high-field MRI device was built at the Inselspital.
After surgery
Histology
After fine tissue (histological) and molecular genetic examination of the removed tissue, the further procedure is discussed by an interdisciplinary team of specialists consisting of neurosurgeons, radiation oncologists and medical oncologists during our weekly tumor board at Inselspital.
Follow-up treatment and controls
In most cases, chemotherapy and radiation are not necessary. Close follow-up with MRI every 3-6 months allows early detection and treatment of tumor recurrence. This strategy is particularly indicated for younger patients under 40–50 years of age after complete removal of the tumor.
In some cases, combined chemotherapy and radiation therapy may be appropriate. These include:
- incomplete removal of the tumor
- older patient age
- very large tumor
- neurological deficits in the patient
- tumor with WHO grade 3
- IDH wild-type gliomas
Chemotherapy is preferred for grade II gliomas in order to avoid radiation therapy due to the relatively long life expectancy.
Neurocognitive testing
The aim of the operation is to enable patients to return to their normal lives quickly after surgery. A fundamental part of the treatment concept is neurocognitive testingof individual brain performance before and after treatment.
Before surgery: A baseline is established for various neurocognitive areas such as attention, memory, etc.. Specialized testing here allows for the objectification of subtle deficits that would escape normal neurological examination * * * * * * * *.
After surgery: re-testing is performed so that differences from pre-surgery neurocognitive performance can be consistently recorded and, if necessary, corrected by neurorehabilitative measures. With the help of outpatient or inpatient rehabilitation measures immediately following the operation, optimal reintegration into work and everyday life is achieved *.
Epileptic seizures
Another important factor in the quality of life of patients with low-grade gliomas is the control of epileptic seizures.
After complete tumor resection, up to two-thirds of patients with pre-existing epileptic seizures become seizure-free in the first year after surgery *. The likelihood of seizure freedom is higher if the tumor could be removed as completely as possible and the epilepsy had not previously occurred for more than one year.
If seizures suddenly increase again after a seizure-free interval, this may be an indication of tumor recurrence.
What if surgery is not possible?
If a tumor cannot be operated on because of its location in a functionally important center or because of its diffuse extension, at least a biopsy should be performed to examine the tissue and confirm the diagnosis.
Biopsy
In a biopsy, a thin needle is inserted precisely into the tumor through a small hole and a tissue sample is taken. The risk of complications from a biopsy is very low, with threatening bleeding occurring in only about 1% of cases.
A more common problem, however, is misgraduation of tumors in up to a quarter of cases *: Because a glioma can be very heterogeneously composed of cells of different WHO grades, biopsy of a less malignant portion leads to the incorrect diagnosis of a low-grade glioma, when in fact it is a higher-grade aggressive glioma.
To mitigate the rate of misdiagnosis, we routinely use techniques such as MR spectroscopy or FET-PET in biopsies.
As soon as an exact diagnosis is available after the biopsy, the tumor is further treated with radiation and chemotherapy.
In individual cases, chemotherapy may make sense before subsequent surgery for an inoperable low-grade glioma. Chemotherapy can push the tumor back so far that an inoperable tumor becomes an operable tumor *. However, this is rather an exception and is not a standard procedure.
What if the tumor recurs?
Further treatment in case of tumor progression depends on various factors:
- neurological condition of the patient
- temporal dynamics
- molecular tumor markers
- therapies already administered
The therapy of choice in the case of recurrence, i.e. a growing tumor, is again surgical removal.
The brain basically has the ability to shift functions from one area of the brain at risk from the tumor to another area of the brain. This is called plasticity. Therefore, it is entirely possible to remove the tumor in a second surgery from a site where brain functions were still present in the first surgery.
Radiation and/or chemotherapy are considered when at least part of the tumor can no longer be operated on. The choice of chemotherapy depends on the genetic character of the tumor.
Chemotherapy
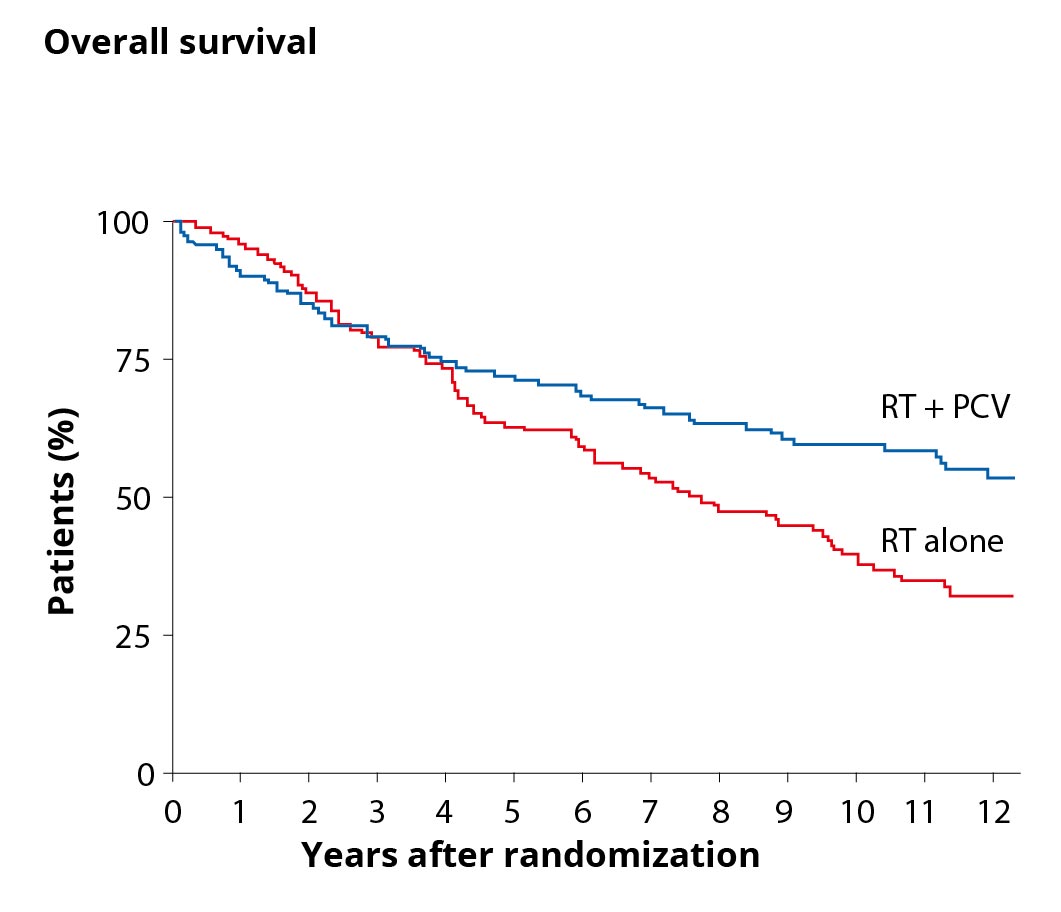
An international study has demonstrated that patients with WHO grade II glioma and the above risk factors benefit from chemotherapy in addition to radiation *.
The graph on the right compares the overall survival of patients with WHO grade II tumors and additional risk factors in two curves. The blue curve represents the overall survival of patients after combined therapy of radiation (RT for radiotherapy) and chemotherapy (PCV) and shows a higher overall survival than the red curve with patients who had radiotherapy as sole therapy.
Chemotherapy cannot be given as often as desired, however, because tumors often develop resistance to chemotherapy. For this reason, it is usually used only in cases of risk factors or malignant degeneration of the glioma.
- Chemotherapeutics
The chemotherapeutic agents used are temozolomide (trade name Temodal®) or PCV. PCV stands for the combination of 3 substances (procarbazine, lomustine and vincristine) and is usually used for oligodendrogliomas with 1p19q codeletion. Temozolomide is more commonly used for diffuse astrocytomas with ATRX mutation.
Radiation therapy
Irradiation is usually given in 30 sessions with a dose of 1.8 Gy each, up to a total dose of 54 Gy. The most common side effects of irradiation are fatigue, nausea, skin irritation, and hair loss. Irradiation also has the disadvantage that years after treatment, long-term brain damage such as leukencephalopathy or general deterioration of brain function may occur.
The EORTC-22845 trial demonstrated that early versus later irradiation does not confer an advantage in terms of overall survival *. Early irradiation only improves progression-free (relapse-free) survival, but not overall survival.
Full radiotherapy can also be given only once. For this reason, it is usually used only in cases of risk factors or malignant degeneration of the glioma.
Complementary medicine
Consultation about complementary medicine therapy options is available upon request at the Institute of Complementary and Integrative Medicine at Inselspital. We can refer you to the consultation hours. The Institute's services include anthroposophically extended medicine, phytotherapy, nutritional counseling, art therapies, eurythmy therapy, and lifestyle and self-enhancement counseling.
Special case: Diffuse astrocytoma without IDH mutation (IDH wild type)
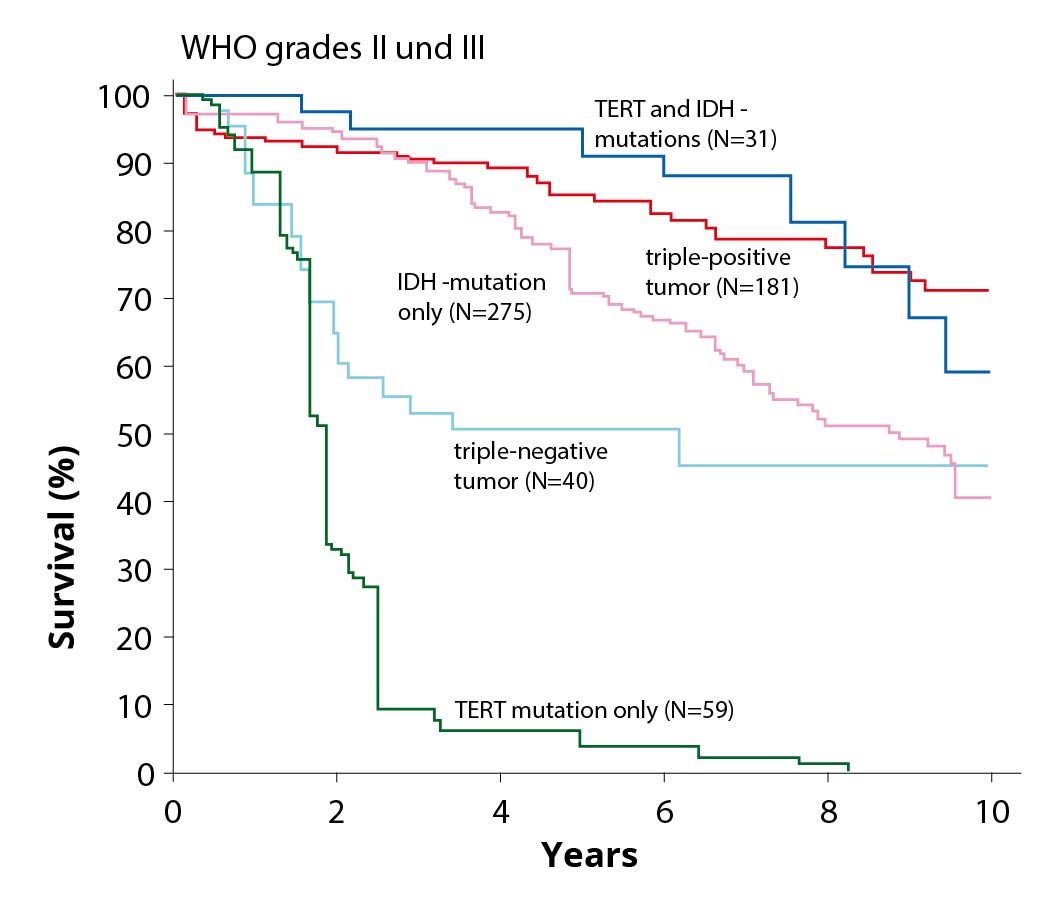
The genetic markers in low-grade gliomas are extremely important because tumors with a different growth behavior "hide" among them.
Diffuse astrocytomas WHO grade II without IDH mutation (IDH wild type) represent such a special case. Although these are classified as grade II by WHO, they behave biologically differently, often show more aggressive growth, and are associated with shorter survival *. Therefore, these tumors should also be followed up with radiation and chemotherapy after complete resection.
- Genetic markers IDH wild type
The typical genetic signature of diffuse astrocytoma with IDH mutation is an alteration in the p53 and ATRX genes. In contrast, diffuse astrocytomas without IDH mutation (IDH wild type) show alterations in the tumor suppressor genes of PTEN, NF1 and CDKN2A as well as in the gene for the EGF receptor and in the TERT gene *.
The product of the TERT gene is an enzyme that prevents the progressive shortening of chromosome ends during rapid cell division. This delays the senescence of a cell.
The EGF receptor picks up signals from a cell's environment that mediate a cell's growth and division. The latter two genes are typically overactive in IDH wild-type glioma.
- Why operate right away if the tumor is growing slowly?
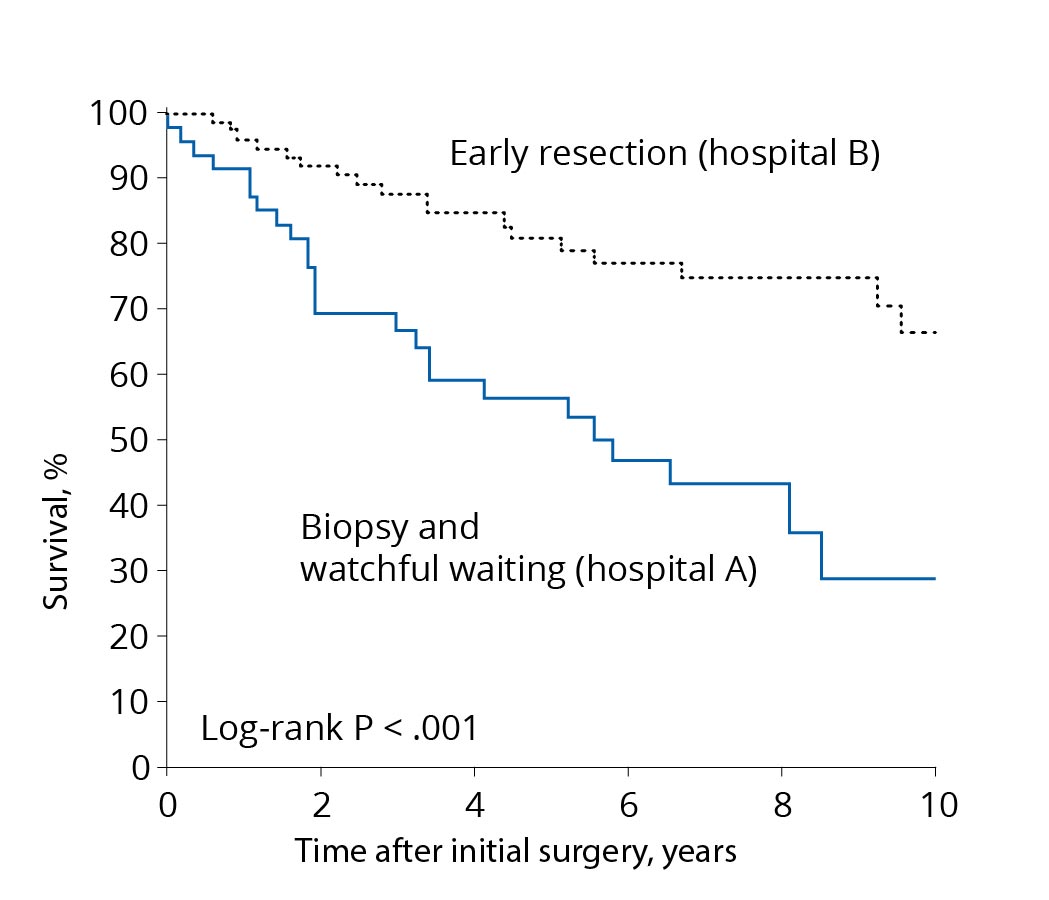
Comparison of treatment concepts. A treatment concept with early resection (black curve) leads to longer survival than a concept favoring biopsy only or a wait-and-see approach (blue curve). Source: According to Jakola et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 2012; 308(18):1881-8. In the past, low-grade gliomas tended to be treated with a wait-and-see approach and operated on late. This had several reasons. First, most patients were still doing very well at the time of diagnosis. In addition, it was rarely possible to remove the tumor completely, as technological development was not yet as advanced as it is today. And also the modern methods of monitoring brain functions and avoiding deficits during surgery were not yet very mature.
The modern strategy of very early surgery is based on relatively recent scientific findings. A comparison of survival times of patients from two university hospitals with two different but consistently followed treatment concepts has shown a clear survival advantage in favor of the patients operated on at an early stage. Follow-up over years or biopsy followed by chemotherapy or radiation without surgery resulted in more frequent malignant transformation and faster disease progression *.
The slow growth behavior of these tumors makes it possible to make the decision to operate carefully and without haste. Surgery should be performed within a few months after diagnosis.
- Where should I have the surgery done?
Gliomas should be operated on in a neuro-oncology center with a large number of cases, proven experience, state-of-the-art technology and genuine interdisciplinary cooperation. Since the term "center of excellence" is not protected, it is worthwhile to take a closer look at the criteria that are really relevant for the evaluation of a hospital:
- Comparison of the official numbers of operated gliomas based on the statistics of the hospital or the public statistics of the federal government.
- Number of national and international publications, i.e. publications of the hospital's own results in scientific journals on the subject of gliomas.
- Confirmation by official certification according to the criteria of the ISO standards (ClarCert) and the German Cancer League (OnkoZert). All disciplines involved in the tumor board – oncology, radiation oncology, neuroradiology, neurology and accompanying subjects – must meet high quality standards and are true cooperating partners.
- Glioma therapy as a scientific and clinical focus, visible in our own studies, in the development of special techniques and methods, in our own database for recording individual results and treatment quality, and in a permanent tumor team with specialized nursing and physicians.
In the Department of Neurosurgery at Inselspital, we fulfill the above-mentioned requirements for a neuro-oncology center and offer you even more!
- What are my chances of recovery?
Tumor cells of gliomas unfortunately tend to spread to the surrounding area of the tumor core at a very early stage. How many cells move away from the main tumor in this process depends on the size of the tumor and its molecular characteristics. For small tumors and favorable tumor characteristics, it may be possible for supramarginal surgery to remove more than 99.9% of the tumor cells, leaving only a few behind. Since not all of these residual cells grow again, there are patients in whom the tumor does not recur for decades or even not at all.
This is why gliomas are referred to as long-term disease control rather than cure. This long-term control is also the subject of current studies. The most important factors influencing tumor recurrence so far are MRI-T2 or FLAIR-complete tumor removal and favorable molecular characteristics of the tumor (1p19q deleted, favorable methylation).
- Will neurological deficits occur after surgery?
In more than 50% of all astrocytomas and oligodendrogliomas, critical, so-called "eloquent" functions of the brain are located in close proximity (less than 10 mm) to the tumor. Preservation of these eloquent functions is always a priority because neurological deficits can accelerate disease progression and lead to dysfunction. Thus, the goal of removing the tumor supramarginally, i.e., with a safety zone, must be subordinate to preserving function.
High-end neurophysiological monitoring is a focus of our clinic, in which we are among the world's leading centers and are continuously involved in further developments. At Inselspital, for example, we have developed a special "Bern Concept" of neuromonitoring with continuous dynamic mapping to prevent paralysis. For tumors near the motor pathway, permanent deficits occur in only 3–5% of our patients. This is one of the lowest numbers for motor deficits in the world!
Only with the help of specialized mapping or functional mapping can surgery be performed precisely to the critical safe distance to eloquent function. This makes supramarginal surgery possible in many astrocytomas and oligodendrogliomas, where without mapping the neurosurgeon would have either stopped removing the tumor too early or already damaged function. With this new and innovative techniques, we achieve sparing or recovery of function in > 90% of all operations in Bern.
- What can I do in addition to standard therapy?
There are a number of factors that contribute to a more successful tumor therapy and thus represent a valuable addition to standard therapy. The goals are
- a faster recovery of our patients
- a shorter stay in hospital
- more patient autonomy
- more safety during treatment
- more quality of life
- a positive effect on tumor control
At Inselspital, we have developed a treatment protocol that takes all these positive factors into account: the so-called OPTIMISST protocol. OPTIMISST stands for "OPTIMIzed Standard and Supportive Therapy".
- What do I have to consider after the surgery?
For most of our patients, we aim for almost the same activity level as before surgery already for the day after surgery, according to the Bern OPTIMISST protocol. However, in case of larger tumors or eloquent location of the operated tumor, about 1–2 months after surgery should be planned for recovery. In this case, it is important to create optimal and stress-free conditions within the family and with the employer for the time after the operation.
In particularly difficult surgeries, deficits in speech, strength, comprehension or other abilities may occur, which in 95% of cases are only temporary. Intensive neurorehabilitation is essential, especially for patients with postoperative deficits, so that the impaired functions recover more quickly.
Tissue diagnosis is available at the latest one week after surgery, and molecular diagnosis after another week. At this point, individual planning of the further procedure (observation or further therapy?) takes place in the tumor board. In special cases, special therapy options or studies are discussed in a conference call with Prof. Roger Stupp from Northwestern University in Chicago, USA.
- How long and how often do I need to have an MRI exam?
When an astrocytoma or oligodendroglioma is diagnosed, MRI follow-up is usually required for life. However, the interval for imaging can be adjusted. The standard times are as follows:
Every 3 months:
- after surgery
- for WHO grade III
- in case of visible tumor remnants
- for IDH wild-type tumors
- in case of PET-active tumor remnants
Every 3–12 months:
- depending on tumor type
- depending on molecular profile
- depending on resection grade
- depending on previous tumor-free period
- depending on progression-free interval
- What happens if I get pregnant? Will the tumor grow faster then?
You should discuss this question with your attending physician in the specific case. Here is some important information:
- To the best of our knowledge, pregnancy for glioma is not a proven risk factor for tumor growth. However, as always, there are individual case reports to the contrary in medicine. Termination of pregnancy in the face of a glioma diagnosis is not recommended based on current knowledge.
- Based on a diagnosis of astrocytoma or oligodendroglioma, the likelihood of tumor growth over the next 3–15 years is relatively high. Surgery or other therapies will be required. The patient and her family need to be aware of this burden.
- Prior chemotherapy may carry an increased risk of mutations and malformations. In these cases, the pregnancy is formally classified as a high-risk pregnancy and closely monitored.
- If possible, a routine MRI scan should be avoided during pregnancy.
- Will my tumor grow again?
Depending on the characteristics and grade of the tumor, it may take months (IDH wild type, grade III astrocytoma) to decades(most favorable case, 1p19q codeletion, other optimal factors) for remaining cells to re-form a visible tumor. This progression is detected in routine MRIs.
If tumor growth resumes, we again clearly recommend resection. Further growth and the likelihood of malignant degeneration to a higher WHO grade increase with tumor volume and time. Often the re-growing tumor also moves closer to eloquent brain areas – specialized functional mapping is therefore usually indispensable.
- What is my prognosis and life expectancy?
Many patients with low-grade gliomas have a good quality of life and work in their learned profession without major limitations in everyday life. Life expectancy varies greatly and depends on many factors.
Positive factors
One important factor is the extent of resection. Patients with a complete resection have a significantly better survival prognosis and fewer malignant degenerations.
We know from large studies that a young age of the patient (under 40–50 years) also has a positive influence on the prognosis.
Another important factor is the absence of neurological deficits. Thus, patients who have epileptic seizures as their only symptom have a better prognosis *.
Negative factors
A negative factor for survival is a large tumor volume of > 4 cm *.
Molecular markers
Patients with IDH mutation have a better prognosis than those without IDH mutation.
Patients with IDH mutation and additional 1p/19q codeletion have the best prognosis of all known subgroups.
Life expectancy
In half of the patients with astrocytomas, IDH and ATRX mutation it is about 7–10 years, in the other half it is shorter or longer.
In patients with oligodendrogliomas, IDH mutation and 1p/19q codeletion it is usually more than 10–15 years, without malignant transformation even several decades.
-
Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, Solheim O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012 Nov 14;308(18):1881-8. doi: 10.1001/jama.2012.12807.
-
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008 Mar 10;26(8):1338-45. doi: 10.1200/JCO.2007.13.9337.
-
Duffau H. Is supratotal resection of glioblastoma in noneloquent areas possible? World Neurosurg. 2014 Jul-Aug;82(1-2):e101-3. doi: 10.1016/j.wneu.2014.02.015. Epub 2014 Feb 15.
-
Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within "noneloquent" areas in the left dominant hemisphere: toward a "supratotal" resection. Clinical article. J Neurosurg. 2011 Aug;115(2):232-9. doi: 10.3171/2011.3.JNS101333. Epub 2011 May 6.
-
Gil-Robles S, Duffau H. Surgical management of World Health Organization Grade II gliomas in eloquent areas: the necessity of preserving a margin around functional structures. Neurosurg Focus. 2010 Feb;28(2):E8. doi: 10.3171/2009.12.FOCUS09236.
-
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro Oncol. 2016 Oct 1;18(suppl_5):v1-v75. doi: 10.1093/neuonc/now207.
-
Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, Berger MS. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008 Feb;108(2):227-35. doi: 10.3171/JNS/2008/108/2/0227.
-
Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg. 2011 Nov;115(5):948-65. doi: 10.3171/2011.7.JNS101238.
-
Potts MB, Smith JS, Molinaro AM, Berger MS. Natural history and surgical management of incidentally discovered low-grade gliomas. J Neurosurg. 2012 Feb;116(2):365-72. doi: 10.3171/2011.9.JNS111068. Epub 2011 Oct 14.
-
Pallud J, Fontaine D, Duffau H, Mandonnet E, Sanai N, Taillandier L, Peruzzi P, Guillevin R, Bauchet L, Bernier V, Baron MH, Guyotat J, Capelle L. Natural history of incidental World Health Organization grade II gliomas. Ann Neurol. 2010 Nov;68(5):727-33. doi: 10.1002/ana.22106.
-
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987 Jun;66(6):865-74. doi: 10.3171/jns.1987.66.6.0865.
-
Pallud J, Varlet P, Devaux B, Geha S, Badoual M, Deroulers C, Page P, Dezamis E, Daumas-Duport C, Roux FX. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology. 2010 May 25;74(21):1724-31. doi: 10.1212/WNL.0b013e3181e04264.
-
Bauman G, Fisher B, Watling C, Cairncross JG, Macdonald D. Adult supratentorial low-grade glioma: long-term experience at a single institution. Int J Radiat Oncol Biol Phys. 2009 Dec 1;75(5):1401-7. doi: 10.1016/j.ijrobp.2009.01.010. Epub 2009 Apr 22.
-
Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006 Mar 15;106(6):1358-63. doi: 10.1002/cncr.21733.
-
Duffau H, Taillandier L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. Neuro Oncol. 2015 Mar;17(3):332-42. doi: 10.1093/neuonc/nou153. Epub 2014 Aug 2.
-
Pedersen CL, Romner B. Current treatment of low grade astrocytoma: a review. Clin Neurol Neurosurg. 2013 Jan;115(1):1-8. doi: 10.1016/j.clineuro.2012.07.002. Epub 2012 Jul 21.
-
Piepmeier JM. Current concepts in the evaluation and management of WHO grade II gliomas. J Neurooncol. 2009 May;92(3):253-9. doi: 10.1007/s11060-009-9870-z. Epub 2009 Apr 9.
-
Soffietti R, Baumert BG, Bello L, Von Deimling A, Duffau H, Frénay M, Grisold W, Grant R, Graus F, Hoang-Xuan K, Klein M, Melin B, Rees J, Siegal T, Smits A, Stupp R, Wick W. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010 Sep;17(9):1124-1133. doi: 10.1111/j.1468-1331.2010.03151.x.
-
Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, Bendszus M, Reijneveld JC, Dhermain F, French P, Marosi C, Watts C, Oberg I, Pilkington G, Baumert BG, Taphoorn MJB, Hegi M, Westphal M, Reifenberger G, Soffietti R, Wick W; European Association for Neuro-Oncology (EANO) Task Force on Gliomas. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017 Jun;18(6):e315-e329. doi: 10.1016/S1470-2045(17)30194-8. Epub 2017 May 5. Erratum in: Lancet Oncol. 2017 Nov;18(11):e642.
-
Capelle L, Fontaine D, Mandonnet E, Taillandier L, Golmard JL, Bauchet L, Pallud J, Peruzzi P, Baron MH, Kujas M, Guyotat J, Guillevin R, Frenay M, Taillibert S, Colin P, Rigau V, Vandenbos F, Pinelli C, Duffau H; French Réseau d'Étude des Gliomes. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013 Jun;118(6):1157-68. doi: 10.3171/2013.1.JNS121. Epub 2013 Mar 15.
-
Nikas DC, Bello L, Zamani AA, Black PM. Neurosurgical considerations in supratentorial low-grade gliomas: experience with 175 patients. Neurosurg Focus. 1998 Apr 15;4(4):e4. doi: 10.3171/foc.1998.4.4.7.
-
Peraud A, Ansari H, Bise K, Reulen HJ. Clinical outcome of supratentorial astrocytoma WHO grade II. Acta Neurochir (Wien). 1998;140(12):1213-22. doi: 10.1007/s007010050241.
-
Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008 Apr;62(4):753-64; discussion 264-6. doi: 10.1227/01.neu.0000318159.21731.cf.
-
van Veelen ML, Avezaat CJ, Kros JM, van Putten W, Vecht C. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998 May;64(5):581-7. doi: 10.1136/jnnp.64.5.581.
-
McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, Olivi A, Brem H, Quinoñes-Hinojosa A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008 Oct;63(4):700-7; author reply 707-8. doi: 10.1227/01.NEU.0000325729.41085.73
-
Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, Wijesekera O, Olivi A, Rahman M, Quinones-Hinojosa A. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014 Jul-Aug;82(1-2):e257-65. doi: 10.1016/j.wneu.2014.01.019. Epub 2014 Feb 6.
-
Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001 Nov;95(5):735-45. doi: 10.3171/jns.2001.95.5.0735.
-
Nakamura M, Konishi N, Tsunoda S, Nakase H, Tsuzuki T, Aoki H, Sakitani H, Inui T, Sakaki T. Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology. 2000 Feb;58(2):108-16. doi: 10.1159/000012087.
-
Nicolato A, Gerosa MA, Fina P, Iuzzolino P, Giorgiutti F, Bricolo A. Prognostic factors in low-grade supratentorial astrocytomas: a uni-multivariate statistical analysis in 76 surgically treated adult patients. Surg Neurol. 1995 Sep;44(3):208-21; discussion 221-3. doi: 10.1016/0090-3019(95)00184-0.
-
De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012 Jul 10;30(20):2559-65. doi: 10.1200/JCO.2011.38.4818. Epub 2012 Apr 23.
-
Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, Lopes M, Mitchell MC, Roche S, Muller JC, Bitar A, Sichez JP, van Effenterre R. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003 Apr;98(4):764-78. doi: 10.3171/jns.2003.98.4.0764.
-
Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg. 2008 Sep;109(3):461-71. doi: 10.3171/JNS/2008/109/9/0461.
-
Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004 Mar;100(3):369-75. doi: 10.3171/jns.2004.100.3.0369.
-
Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014 May;120(5):1015-24. doi: 10.3171/2014.1.JNS13909. Epub 2014 Mar 14.
-
Chacko AG, Thomas SG, Babu KS, Daniel RT, Chacko G, Prabhu K, Cherian V, Korula G. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin Neurol Neurosurg. 2013 Mar;115(3):329-34. doi: 10.1016/j.clineuro.2012.10.022. Epub 2012 Nov 21.
-
Spena G, Garbossa D, Panciani PP, Griva F, Fontanella MM. Purely subcortical tumors in eloquent areas: awake surgery and cortical and subcortical electrical stimulation (CSES) ensure safe and effective surgery. Clin Neurol Neurosurg. 2013 Sep;115(9):1595-601. doi: 10.1016/j.clineuro.2013.02.006. Epub 2013 Mar 5.
-
Campanella F, Shallice T, Ius T, Fabbro F, Skrap M. Impact of brain tumour location on emotion and personality: a voxel-based lesion-symptom mapping study on mentalization processes. Brain. 2014 Sep;137(Pt 9):2532-45. doi: 10.1093/brain/awu183. Epub 2014 Jul 15.
-
Shields LB, Choucair AK. Management of low-grade gliomas: a review of patient-perceived quality of life and neurocognitive outcome. World Neurosurg. 2014 Jul-Aug;82(1-2):e299-309. doi: 10.1016/j.wneu.2014.02.033. Epub 2014 Feb 19.
-
Aaronson NK, Taphoorn MJ, Heimans JJ, Postma TJ, Gundy CM, Beute GN, Slotman BJ, Klein M. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011 Nov 20;29(33):4430-5. doi: 10.1200/JCO.2011.35.5750. Epub 2011 Oct 17.
-
Carlesimo GA. Memory disorders in patients with cerebral tumors. J Neurooncol. 2012 Jun;108(2):253-6. doi: 10.1007/s11060-012-0825-4. Epub 2012 Feb 21.
-
Klein M, Heimans JJ, Aaronson NK, van der Ploeg HM, Grit J, Muller M, Postma TJ, Mooij JJ, Boerman RH, Beute GN, Ossenkoppele GJ, van Imhoff GW, Dekker AW, Jolles J, Slotman BJ, Struikmans H, Taphoorn MJ. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002 Nov 2;360(9343):1361-8. doi: 10.1016/s0140-6736(02)11398-5. Erratum in: Lancet. 2011 May 14;377(9778):1654.
-
Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004 Mar;3(3):159-68. doi: 10.1016/S1474-4422(04)00680-5.
-
Teixidor P, Gatignol P, Leroy M, Masuet-Aumatell C, Capelle L, Duffau H. Assessment of verbal working memory before and after surgery for low-grade glioma. J Neurooncol. 2007 Feb;81(3):305-13. doi: 10.1007/s11060-006-9233-y. Epub 2006 Aug 31.
-
Gehring K, Sitskoorn MM, Gundy CM, Sikkes SA, Klein M, Postma TJ, van den Bent MJ, Beute GN, Enting RH, Kappelle AC, Boogerd W, Veninga T, Twijnstra A, Boerman DH, Taphoorn MJ, Aaronson NK. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009 Aug 1;27(22):3712-22. doi: 10.1200/JCO.2008.20.5765. Epub 2009 May 26.
-
Muragaki Y, Chernov M, Maruyama T, Ochiai T, Taira T, Kubo O, Nakamura R, Iseki H, Hori T, Takakura K. Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim Invasive Neurosurg. 2008 Oct;51(5):275-9. doi: 10.1055/s-0028-1082322. Epub 2008 Oct 14.
-
Blonski M, Taillandier L, Herbet G, Maldonado IL, Beauchesne P, Fabbro M, Campello C, Gozé C, Rigau V, Moritz-Gasser S, Kerr C, Rudà R, Soffietti R, Bauchet L, Duffau H. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J Neurooncol. 2012 Jan;106(2):353-66. doi: 10.1007/s11060-011-0670-x. Epub 2011 Jul 22.
-
Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K, Brachman D, Suh JH, Schultz CJ, Bahary JP, Fisher BJ, Kim H, Murtha AD, Bell EH, Won M, Mehta MP, Curran WJ Jr. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016 Apr 7;374(14):1344-55. doi: 10.1056/NEJMoa1500925.
-
van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005 Sep 17-23;366(9490):985-90. doi: 10.1016/S0140-6736(05)67070-5. Erratum in: Lancet. 2006 Jun 3;367(9525):1818.
-
Chang EF, Smith JS, Chang SM, Lamborn KR, Prados MD, Butowski N, Barbaro NM, Parsa AT, Berger MS, McDermott MM. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008 Nov;109(5):817-24. doi: 10.3171/JNS/2008/109/11/0817.
-
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, ... Zhang J. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015 Jun 25;372(26):2481-98. doi: 10.1056/NEJMoa1402121. Epub 2015 Jun 10.
-
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O'Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015 Jun 25;372(26):2499-508. doi: 10.1056/NEJMoa1407279. Epub 2015 Jun 10.